NSF REU
The Ole Miss Physical chemistry Research Program seeks applicants for a summer Research Experience for Undergraduates (REU) program funded by CHE-1156713, CHE-1460568, CHE-1757888 and CHE-2150352. Ten non-University of Mississippi students who have completed their freshman year of college and who have not yet graduated can participate fully in the Ole Miss Physical Chemistry Research Program activities and work on a research project under the direction of a faculty advisor. External (non-UM) student participants will receive a $6,000 stipend, a housing and meal plan for ten weeks, and travel assistance. Undergraduate student participants must be citizens or permanent residents of the United States or its possessions. For more information, contact program director Dr. Nathan I. Hammer at nhammer@olemiss.edu. Click on "Faculty" on the menu bar for a list of participating faculty and click on "Example Research Projects" to the right for example research projects.


Example Research Projects
Spectroscopic Studies of Biological Building Block Interactions and the Photophysical Properties of New Emissive Molecules
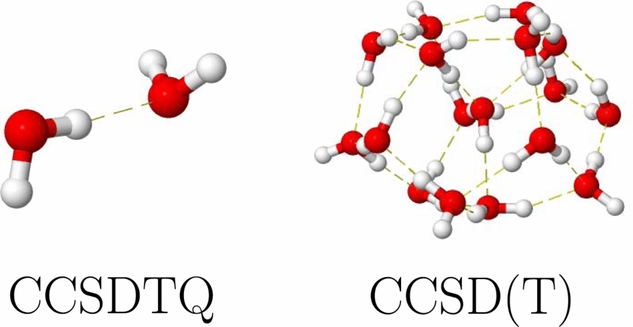
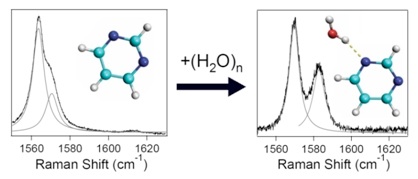
The Hammer Research Group studies the fundamental physical properties of interacting biologically-relevant building blocks and newly-developed nanoscale molecular systems using laser-based vibrational and electronic spectroscopies. Their central goal is to help model these complex systems through comparison of experimental spectroscopic observables to the results of theoretical predictions. To accomplish this goal, members of the Hammer Research Group work closely with theoretical and synthetic collaborators in the design and study of these systems. REU students will work alongside Prof. Hammer and his current graduate and undergraduate students in established NSF-funded research areas but will have their own unique projects to be completed by the end of the summer. Each summer, one student will study the fundamental spectroscopic properties of an important biological building block interacting with water and the other student will study the fundamental properties of newly-developed materials that either have unique emissive or architectural properties. Both projects will be collaborative in nature with computational mentoring from either co-PI Prof. Gregory Tschumper or senior personnel Prof. Robert Doerksen. In the first project, the student will employ Raman and SERS spectroscopies to study a biological building block or biologically relevant small molecule (we have been studying TMAO and pyrimidine most recently) and its interactions with water (as shown in the figure) or other solvents. This student will then simulate the properties of the interacting system and its vibrational frequencies using the quantum mechanical packages learned as part of the REU program. In the second project, the student will study the photophysical properties of newly-developed emissive materials using laser-based Raman, fluorescence, and single molecule spectroscopies. As in the first project, the student will compare their experimental results to theoretical predictions. Prof. Hammer is currently working with a number of synthetic collaborators, including senior personnel Prof. T. Keith Hollis, Prof. Daniell Mattern (Ole Miss), Prof. Gary Gray (University of Alabama-Birmingham), and Prof. Hemali Rathnayake (Western Kentucky University). The specific system under study will change year to year. These two projects have proven to be extremely successful for researchers over the past three years in the Hammer Research Group with a number of resulting publications, including two with undergraduates appearing as first author. Both students will receive laser safety training and will be under the direct supervision of Prof. Hammer.
Using Ionic Liquids for Drug Delivery
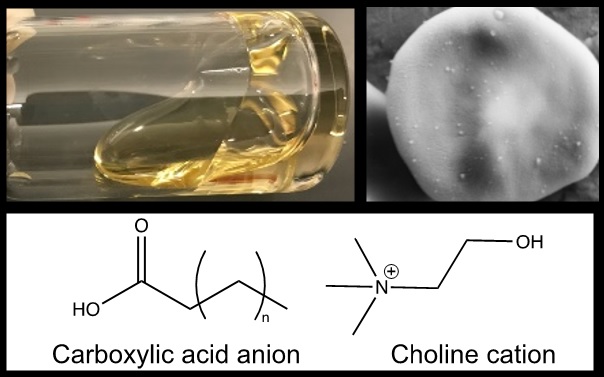
The Tanner Lab works at the interface of physical chemistry and bioengineering, using a physical chemistry toolkit to tackle biomedical problems. Of particular interest is nanoparticle drug delivery, where fragile or toxic pharmaceuticals are packaged inside nanoparticles before being injected into the bloodstream. However, the vast majority (>99%) of the injected nanoparticles do not reach their intended destination, which hampers their clinical progress. The Tanner Lab develops ionic liquids, which consist of biocompatible anions and bulky cations that are liquid less than 100 °C, to coat the nanoparticle surfaces and modulate the interactions between the nanoparticle and the body. As an example, they discovered that certain ionic liquids are able to hitchhike onto red blood cells in situ, and are now working to understand the chemistry of that interaction. REU participants who join the Tanner Lab will work within a collaborative, diverse, and interdisciplinary environment to investigate biochemical interactions at the nanoscale. They will learn how to design and synthesize ionic liquids, create and coat nanoparticles, and investigate their interactions with biomaterials such as blood, mucus, and skin. They will gain new perspectives on the vital role physical chemistry plays in understanding the human body.
Computational Astrochemistry
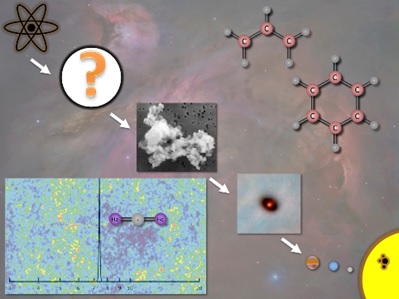
Undergraduate students working with Dr. Fortenberry and his group will get the opportunity to explore molecular structures uncommon under terrestrial conditions and compute accurate spectral data for their detection in support of the Stratospheric Observatory for Infrared Astronomy or the upcoming James Webb Space Telescope. Most of the carbon in the Universe is either tied up in carbon monoxide or polycyclic aromatic hydrocarbons (PAHs). Hence, the rest of the astrochemists’ periodic table is providing motivation for exploration of molecules rarely examined previously or those that are completely new to science. These can include noble gas molecules from potentially the age of the Universe before stars formed; inorganic species that may be constituents of larger crystals and rocks showing up during the formation or destruction of rocky planets; radicals, anions, or cations that are perpetuated in cold molecular clouds; or even PAHs present in nearly every astrophysical region. Once viable molecular candidates are chosen from these sets, accurate anharmonic vibrational frequencies and spectroscopic constants are computed such that observatories can examine the heavens for their possible existence.
Quantitative and Qualitative Analyses
The Boyd Research Group focuses on chemical education and environmental chemistry. Specific topics and techniques will vary yearly however, REU students will explore topics such as such as student perception of real-world relevance of physical chemistry topics such as quantum mechanics, the design and implementation of engaged learning activities (e.g., virtual reality, research experiences, place-based learning) into physical chemistry, and recruitment and retention within chemistry. REU students working with Boyd will be exposed to both quantitative and qualitative analysis techniques. Environmental studies may include trace metal analysis of samples collected in Mississippi exposure to students of a variety of spectroscopic techniques.

Computational Studies of the Structures of Novel Nanomaterial/RNA Complexes
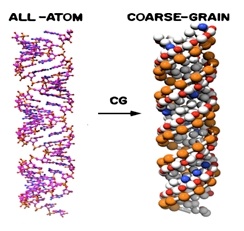
The Wadkins Research Group studies the structures and properties of large biologically relevant molecules both theoretically and experimentally. One long-term project involves modeling the structure of novel nanomaterial/RNA complexes that incorporate novel RAFT polymers. This is a collaborative project involving faculty members from three universities (Ole Miss, University of Mississippi Medical Center in Jackson, and the University of Southern Mississippi). These new hybrid nanomaterials developed at USM have been shown to have the requisite properties necessary for cell delivery. Prof. Wadkins will host one REU student in his laboratories per summer to study the properties of these new nanomaterials theoretically. The entire research project is directed at mediating the strength of copolymer/siRNA complexes in order to enhance siRNA release within the cell and eventually lead to superior gene knockdown. The experimental data generated is being utilized to develop computational models and methods for this new class of nanomaterial. Developing models of this system is challenging because the RNA itself is extremely large, and a single RAFT polymer has thousands of atoms as well. To make the modeling problem computationally feasible, the Wadkins Group is employing a technique referred to as “coarse-graining” to reduce the all-atom models to smaller, more tractable models as illustrated in the figure below. The Wadkins Group has already developed the coarse-grain model for the siRNA and the all-atom model of a polymer is now working on coarse-graining the carrier polymer. However, the carrier polymers are not homogeneous in composition, and hence statistical distribution simulations are being performed with the RAFT polymers to determine how the heterogeneity of the polymers affects siRNA binding and release. The REU student each summer will analyze computationally how differing polymer compositions result in different RAFT polymer structures, and how each of these interact with siRNA. In future years, the composition of the nanomaterials will evolve with new synthetic developments at USM.
Computational Studies of Novel Natural Products
The Doerksen Research Group uses computational methods to study natural products. New natural product molecules often are highly flexible (many rotatable single bonds) and generally contain asymmetric carbons. It can be difficult to determine the absolute configuration (AC) of such a molecule; yet it is important to do so since the AC defines the molecule precisely, and hence is useful for identification, in planning for total synthesis of the molecule, and for understanding the mechanism of action of the molecule as it interacts with chiral protein targets. X-ray crystallography can be used to help determine the AC, but it is often impossible to crystallize the molecule. Methods that involve degradation of the molecule, such as the use of Mosher’s ester, are not generally applicable because typically novel natural product molecules are available in miniscule quantities of a few milligrams at most. A solution is to combine spectroscopic analysis of the molecule with accurate ab initio calculations of properties of the molecule that define the response of a molecule to applied polarized light. The optical rotation (OR) and electronic or vibrational circular dichroism (ECD or VCD) experimental data for the natural product can be compared to the calculated data for one or more particular diastereomers of the natural product in order to aid assignment of the AC. Students in Prof. Doerksen’s research group will learn how to do ab initio calculations (as part of the REU training) and perform calculations, including conformational search, to assist the assignment of the absolute configuration for one or more novel natural product molecules discovered by Doerksen’s collaborators in the National Center for Natural Products Research (NCNPR). Such efforts in the Doerksen lab have led to a series of recent papers on AC assignment.
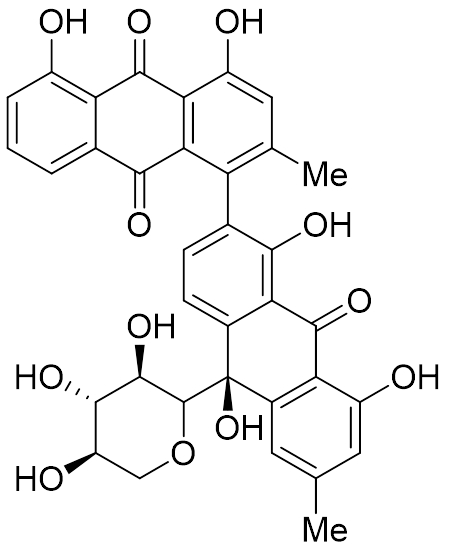
Artificial Metalloenzymes for Electrocatalytic Hydrogen Evolution and CO2 Reduction
Inspired by hydrogenase and CO-dehydrogenase enzymes the Chakraborty Research Group develops artificial metalloenzymes for electrocatalytic hydrogen evolution and CO2 reduction. Metalloenzymes represent some of the best known inorganic catalysts in nature, catalyzing difficult reactions with exceptional efficiency and selectivity. Employing rational protein design and synthetic inorganic chemistry, the selectivity of protein scaffolds is merged with the versatility of inorganic catalysts to design unique biocatalysts for selective and efficient transformations relevant to alternate energy. REU students in this project will gain experience in a wide range of chemistry skills including rational protein design, inorganic synthesis, protein expression and purification, conjugation chemistry, chromatography, UV-visible spectroscopy, circular dichroism spectroscopy, electrochemistry, and catalysis.
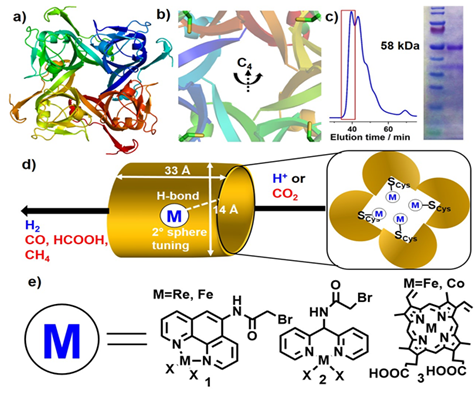
New Fuel Cell Electrolytes
The Ritchie Research Group studies ionic conductivity in H+ ion conducting Fuel Cell electrolytes. Finding new polymeric fuel cell electrolytes is critical to commercializing fuel cells, especially with electrolytes that can conduct H+ ions at temperatures above 120°C. They are currently working to understand how the structure of polymer electrolytes affect the movement of H+ ions (i.e. the mechanism of ionic transport) in the polymer. REU students will synthesize new polymers, use electrochemical techniques to measure their ionic conductivity and the activation barrier to ionic conductivity, and use polymer characterization and rheometry techniques to measure viscosity as a function of the free volume of the polymers. They will also make different high free volume siloxane POSS Cubes with different polymer "tails" to see how the free volume in the tails affect the ionic transport properties. POSS cubes are a well-defined structure that makes comparison between polymers with different side chains easier. Students will prepare PEG-based and PPG-based (polymer)8T8 POSS cube polymers.
Characterizing Microplastic (MP) Pollution
The Cizdziel Group uses microspectroscopy to characterize microplastic (MP) pollution in environmental and biological samples. They develop and apply novel analytical methods to collect, extract, concentrate, detect, and identify microplastics from natural water, air, and biota. The goal is to assess sizes, shapes, and chemical composition of the microplastics to better understand the physicochemical properties, distribution, types, and sources of MPs in the environment and the threat they may pose to both ecosystems and human health. Microplastics are a diverse suite of contaminants consisting of small (less than 5 mm) pieces of synthetic polymers. The different sizes and types of microplastics (and plastic additives) influence their transport, fate, and effects. The ubiquity of microplastics in aquatic ecosystems is of great concern because plastics are persistent, can adsorb toxic chemicals, and are consumed by organisms. These concerns are reflected in the ever-increasing number of papers on microplastics (<500 in 2010 to >5000 in 2020). Students in the Cizdziel group train on advanced chemical instrumentation and conduct exciting research on an emerging global issue, better preparing them for the next steps in their career.

Developing and Understanding New Earth-Abundant Transition Metal Catalysts
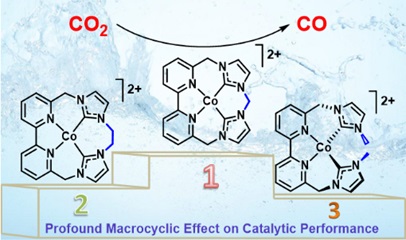
Chemistry in the Jurss Research Group focuses on developing and understanding new earth-abundant transition metal catalysts for reactions relevant to global energy concerns. Energy consumption continues to climb with economic growth and an increasing global population. One of the best candidates for creating a sustainable, carbon-neutral energy economy is sunlight. Artificial photosynthesis aims to store solar energy in the chemical bonds of renewable fuels, such as H2 and CH4, by coupling water oxidation to reductive half reactions (e.g. carbon dioxide reduction). To effectively utilize carbon dioxide and water, better catalysts are needed to mediate these challenging multielectron reactions. Our strategy for converting CO2 and H2O into energy-rich chemical fuels involves the rational design of molecular catalysts with redox-active and/or dinucleating ligands, which enable access to multiple redox equivalents at modest potentials and cooperative bimetallic pathways, respectively. Pendant functional groups in the second-coordination sphere will also be incorporated into catalysts to stabilize intermediates and enhance reactivity. REU students in the Jurss Lab will be involved in the synthesis and investigation of innovative catalysts for renewable energy applications. Electrochemistry and a suite of spectroscopic techniques (NMR, UV-Vis, FTIR, fluorescence, spectroelectrochemistry) will be employed to examine these systems. Raman and transient absorption spectroscopies will also be used in collaboration with the Hammer Group to study the electronic structure, photophysical properties, and reaction mechanisms of these systems.
Developing Tunable Nanomaterials
The primary research thrust in the Li Research Group is the development of molecular nanographenes, which are discrete, structurally precise cut-outs of graphene sheets. These nanocarbon materials, possessing desirable physical properties that are highly tunable through chemical modification, are of great importance for applications in electronics, catalysis and biomedicine. REU students joining the Li group will employ physical organic and supramolecular principles to innovate nanographenes with unique optoelectronic, magnetic, and recognition behaviors for organic electronics and analyte sensing. Alongside traditional benchtop solution-phase chemistry, we are developing solid-state mechanochemical synthesis, a sustainable and efficient method that harnesses mechanical force to drive chemical transformations. Students working with prof. Li on this project will receive hands-on training on organic synthesis as well as materials characterization techniques including NMR, UV-Vis-NIR spectroscopy, cyclic voltammetry, X-ray crystallography and size exclusion chromatography.
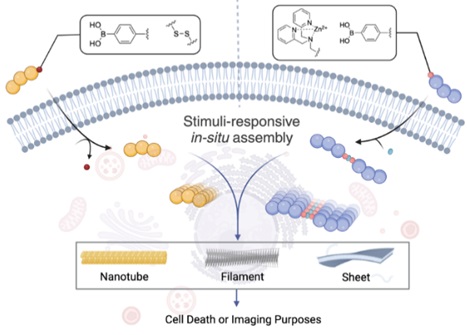
Peptide-Based Materials for Biomedical Applications
The Lou Research Group leverages organic chemistry to develop peptide-based materials for biomedical applications and to address the challenges in the field of nanomedicine. The emerging fields of nanomedicine and biomaterials have the potential to significantly impact human health by improving the diagnosis, prevention, and treatment of diseases. Despite their promise in cancer theranostics, nanomedicines face limitations in deep tumor penetration due to their nanoscale size. Conversely, small molecule drugs exhibit excellent deep penetration features, but are often easily leaked out or expelled from tumor tissues. Therefore, finding a balance between deep tumor penetration and effective tumor-specific accumulation of nanomaterials represents a critical scientific challenge in anticancer drug delivery. REU students joining the Lou lab will engage in projects focused on an in situ self-assembly approach to address this issue. This method involves exogenous low molecular weight molecules spontaneously forming sophisticated nanostructures with distinct biological functions in response to specific physiological and pathological conditions. These nanostructures accumulate and persist at the pathology site, enhancing their efficacy. REU students in the Lou Lab will receive training in various areas, including synthetic organic techniques, instrumentation (NMR spectroscopy, mass spectrometry, UV-Vis, IR, etc.), nanomaterial construction and characterization, and cell-related assays. This comprehensive training will help students expand their career options and prepare them for future scientific endeavors.
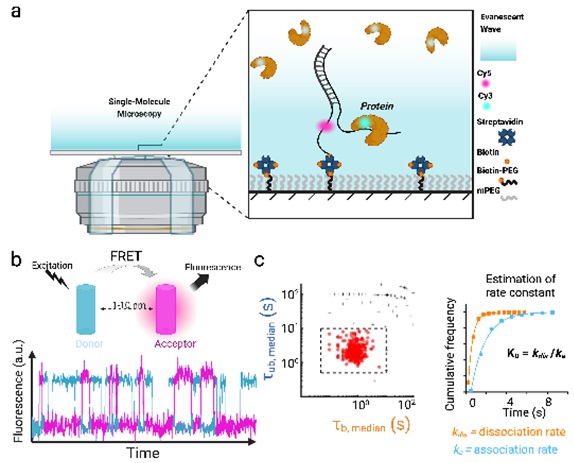
Understanding the biochemical Nature of Cellular Responses
The Ray Research Group investigates the intricate workings of biological systems at the nanoscale, with a particular emphasis on cellular stress response mechanisms. Research focuses on examining how specific DNA and RNA structures enable cells to efficiently allocate resources and adapt to challenging environmental conditions by prioritizing crucial functions. They aim to elucidate the complex interactions between these DNA/RNA structures and various proteins, exploring how they collectively manage cellular demands. The goal is to establish clear connections between structural characteristics and functional responses within cells. To achieve these objectives, they develop and implement cutting-edge single-molecule manipulation and detection techniques, as well as nanoscale engineering approaches. Projects are inherently interdisciplinary, combining methodologies from chemistry, physics, molecular biology, and engineering. As an REU participant in the Ray Group, students will gain hands-on experience in developing highly sensitive detection methods for specific DNA/RNA secondary structures using kinetic fingerprinting techniques. Through these projects, students will gain hands-on experience with a range of advanced research methodologies, including: (1) design and automation of single molecule TIRF microscope, (2) design and optimization of nucleic acid probes, (3) application of single-molecule manipulation and detection methods, (4) use of nanoscale engineering techniques and (5) data analysis and interpretation of kinetic fingerprinting results. This experience will not only provide students with valuable technical skills but also contribute to the broader understanding of cellular stress response mechanisms at the molecular level. By developing more sensitive and specific detection methods for DNA/RNA secondary structures, researchers can gain deeper insights into how these structures interact with proteins and help cells manage resources under stress conditions at the molecular level.
Studying Electrochemical Phenomena at the Nanoscale
Research in the Sundaresan Group focuses on developing and using state-of-the-art electrochemical and optical techniques to study electrochemical phenomena at the nanoscale. Recently, we have developed a new multi-parameter super-resolution optical imaging technique called calcite-assisted localization and kinetics (CLocK) microscopy. This technique enables researchers to obtain the structural anisotropy and orientation of individual nanoparticles by overcoming the fundamental limitations of optical microscopy—the diffraction limit of light. REU students will work on interfacing CLocK microscopy with electrochemical techniques to understand how dynamic changes in the shape of nanoparticle electrocatalysts impact their electrochemical properties. REU students in this project will gain experience in nanoparticle synthesis, various modalities of optical microscopy including CLocK microscopy, electroanalytical techniques such as cyclic voltammetry, amperometry, and scanning electrochemical cell microscopy, as well as image analysis using MATLAB.

REU 2013 Students Funded by CHE-1156713

Sarah Adams
Home College: Mississippi College
Faculty Advisor: Jim Cizdziel
Project Title: "Mercury Concentrations in Mississippi Fish"

Ryan Scott Bowen
Home College: Lipscomb University
Faculty Advisor: Randy Wadkins
Project Title: "Spectroscopic Studies of i-motif Folding and Formation at Neutral pH in the Presence of Crowding Agents"

Sarah Delee
Home College: Mississippi College
Faculty Advisor: Nathan Hammer
Project Title: "Spectroscopic and Computational Investigation of the Effects of Hydrogen Bonding on 2-Aminopyrimidine and Nicotinamide"

Ronnie Funk
Home College: Erskine College
Faculty Advisor: Randy Wadkins
Project Title: "Expression of PCBP-2 in Origami B E. coli and Quantification of PCBP-2 to cytosine rich DNA "
Jessica Gray
Home College: Georgia Southern University
Faculty Advisor: Gregory Tschumper
Project Title: "Basis Set Convergence of Vibrational Frequencies in HF Systems"

Laura Beth Jobe
Home College: Erskine College
Faculty Advisor: Robert Doerksen
Project Title: "Water Pyrimidine Interactions: Application to Protein Inhibitor Complexes"

Sheldon McLetchie
Home College: University of Kentucky
Faculty Advisor: Amal Dass
Project Title: "Gold Nanoparticles"
Linh Nguyen
Home College: Austin Peay State University
Faculty Advisor: Gregory Tschumper
Project Title: "Basis Set Convergence of Vibrational Frequencies in Water Clusters"

Caleb Swain
Home College: Georgia Southern University
Faculty Advisor: Robert Doerksen
Project Title: "Hydrogen Bonding in the Active Site of Cyclin-dependent Kinase 2: A Computational Investigation"

Terri Turpeau
Home College: Southern University and A&M College
Faculty Advisor: Steve Davis
Project Title: "Computational Investigation of a Highly Strained Molecule: Quadricyclene"
REU 2014 Students Funded by CHE-1156713

Jakob Anderson
Home College: Bellhaven University
Faculty Advisor: Gregory Tschumper
Project Title: "Computational Study of Novel Heterocyclic Structures for Dye Sensitized Solar Cells"

Jordan Cauley
Home College: Mississippi College
Faculty Advisor: Nathan Hammer
Project Title: "Spectroscopic and Computational Studies of Urea/TMAO/Water Solutions"

Ashlee Colbert
Home College: Florida A&M University
Faculty Advisor: Robert Doerksen
Project Title: "An Analysis of Water and Pyrimidine-Containing ATP Competitive Inhibitors on GSK-3B: A Computational Study"

Michael Concepción
Home College: Universidad Metropolitana
Faculty Advisor: Robert Doerksen
Project Title: "Computational Analysis of Aminopyrimidine Inhibitors of Protein Kinase GSK-3B"

Arielle Hackel
Home College: Georgia State University
Faculty Advisor: Randy Wadkins
Project Title: "Molecular Modeling of siRNA-Binding Cationic Copolymers"
Spencer Hinton
Home College: University of Tennessee
Faculty Advisor: Gregory Tschumper
Project Title: "C-H•••O and π-Stacking Non-Covalent Interactions in Clusters of Weakly Bound Z-Formic Acid Monomers"

Lawson Lloyd
Home College: College of Charleston
Faculty Advisor: Nathan Hammer
Project Title: "SERS of Azabenzenes in Hydrogen Bonded Networks"

Emily McClary
Home College: Syracuse University
Faculty Advisor: Steven Davis
Project Title: "MCSCF Study of the Ring Opening of 3-Phospho-dihydrobenzvalene: Release of Strain Energy"
Joseph Murphy
Home College: Itawamba Community College
Faculty Advisor: Jared Delcamp
Project Title: "π-Bridges for a Novel Indolizine Dye-Sensitized Solar Cell Donor"

Jocelyn Newman
Home College: University of Alabama
Faculty Advisor: Randy Wadkins
Project Title: "Investigations of Molecular Crowding in I-Motifs"
REU 2015 Students Funded by CHE-1156713

Marjory Clement
Home College: Bellhaven University
Faculty Advisor: Gregory Tschumper
Project Title: "Convergent quantum chemistry for challenging dispersion-dominated non-covalent dimers"
Grant: CHE-1156713

Gerardo Colon
Home College: Universidad Metropolitana
Faculty Advisor: Gregory Tschumper
Project Title: "Energetics of Boryl Enolate/Ketone Adducts"
Grant: CHE-1156713

Valerie Huang
Home College: University of Southern California
Faculty Advisor: Robert Doerksen
Project Title: "Computational study of pyrimidine-containing kinase inhibitors of VEGFR2"
Grant: CHE-1156713

Cameron Lee
Home College: Samford University
Faculty Advisor: Robert Doerksen
Project Title: "A Computational Study of Pyrimidine-Containing Inhibitors of GSK-3B"
Grant: CHE-1156713

Justin Parmely
Home College: Austin Peay State University
Faculty Advisor: Randy Wadkins
Project Title: "The Effects of Ligand Binding to Loops in the DNA I-Motif"
Grant: CHE-1156713

Elliot Taylor
Home College: Mississippi College
Faculty Advisor: Steven Davis
Project Title: "Ring opening of C5SH6 studied using MCSCF techniques"
Grant: CHE-1156713

Hannah Trent
Home College: Itawamba Community College
Faculty Advisor: Nathan Hammer
Project Title: "DMSO-Water Interactions"
Grant: CHE-1156713

Hanfei Wang
Home College: Vanderbilt University
Faculty Advisor: Randy Wadkins
Project Title: "Effects of Mercury (II) Ion on I-Motif Formation by TiM DNA"
Grant: CHE-1156713

Kayla Warren
Home College: Georgia Southern University
Faculty Advisor: Nathan Hammer
Project Title: "Non-Covalent Interactions Involving Trimethlamine N-Oxide (TMAO) and Urea in Water"
Grant: CHE-1156713

Alexa Zylstra
Home College: Mississippi College
Faculty Advisor: Jared Delcamp
Project Title: "Pro-aromatic DSC Dyes with Hagfeldt and Indoline Donors"
Grant: CHE-1156713

Jonathan Ishee
Home College: Mississippi Gulf Coast Community College
Faculty Advisor: Gregory Tschumper
Project Title: "Simple models of host/guest binding interactions in self-heating polymers"
Grant: NSF EPS-1430364
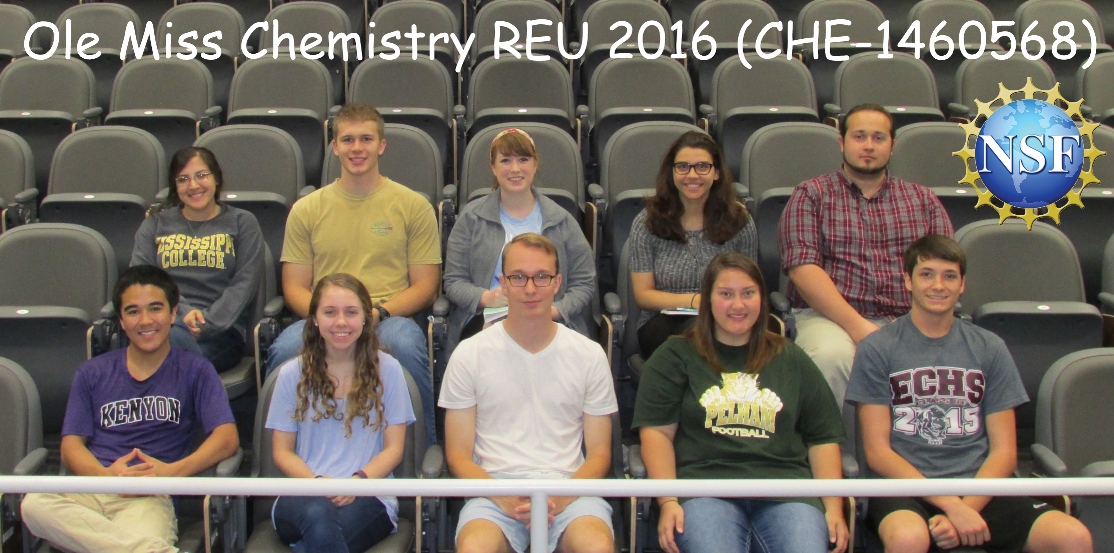
REU 2016 Students

Isabel Bogacz
Home College: St. Lawrence University
Faculty Advisor: Gregory Tschumper
Project Title: "Conformational Energetics of Cyclohexane, THP, and Dioxane"
Grant: NSF IIA-1430364

Shayna Burrage
Home College: Trine University
Faculty Advisor: Amal Dass
Project Title: "Au38(SPh)24 and Aromatic Ligand Exchange"
Grant: NSF EPS-0903787

Zachary Cuny
Home College: Mississippi State University
Faculty Advisor: Robert Doerksen
Project Title: "Molecular Modeling Studies on the Inclusion Complexes of Indomethacin with beta-Cyclodextrin and its Derivatives"
Grant: NSF CHE-1460568

Theodora Leventis
Home College: University of Missouri
Faculty Advisor: Amal Dass
Project Title: "An Investigation into Au25(SPh)18 via Ligand Exchange on Au25(SCH2CH2Ph)18"
Grant: NSF CHE-1460568

Charlotte McBride
Home College: Homes University
Faculty Advisor: Susan Pedigo
Project Title: "Cloning, Expression, and Purification of Proline Mutants in Epithelial and Neural Cadherin"
Grant: NSF CHE-1460568

Amber Morales
Home College: Mississippi College
Faculty Advisor: Jared Delcamp
Project Title: Utilizing CPDT Pi - bridges in Dye-sensitized Solar Cells
Grant: NSF CHE-1460568

Lane Parmely
Home College: Austin Peay State
Faculty Advisor: Randy Wadkins
Project Title: The Effects of Ligand Binding to Loops in the DNA I-Motif
Grant: NIH 1R15CA173667-01A1

Erin Reph
Home College: Bucknell University
Faculty Advisor: Davita Watkins
Project Title: Synthesis of Novel Furan Based Semiconducting Molecules
Grant: NSF CHE-1460568

Jordan Spell
Home College: Western Carolina University
Faculty Advisor: Jonah Jurss
Project Title: Bioinspired O2 Reduction Catalyst
Grant: NSF CHE-1460568

Kimberly Stevens
Home College: University of Kentucky
Faculty Advisor: Nathan Hammer
Project Title: Studies of pyridazine/water mixtures
Grant: NSF CHE-1460568

Daniel Touzeau
Home College: University of Alabama Huntsville
Faculty Advisor: Nathan Hammer & Jared Delcamp
Project Title: Synthesis and Characterization of an Indolizine-based Donor-Acceptor Molecule for Use in Dye Sensitized Solar Cells
Grant: NSF OIA-1539035

Sarah Grace Travis
Home College: Mississippi College
Faculty Advisor: Nathan Hammer
Project Title: "Noncovalent Interactions between Tri-methylamine N-oxide (TMAO), Urea, and Water"
Grant: NSF EPS-0903787

Bryce Wedig
Home College: Kenyon College
Faculty Advisor: Katherine Dooley
Project Title: Reducing Quantum Noise in LIGO: Characterization of an Ultra-Low Loss Polarizing Beam Splitter
Grant: NSF CHE-1460568

Justin Weeks
Home College: Central Alabama Community College
Faculty Advisor: Gregory Tschumper
Project Title: "Hydrogen Bonding in 1,2-Disubstituted-2,3-Epoxy Cyclopentanols"
Grant: NSF CHE-1460568

Spencer Yeager
Home College: Temple University
Faculty Advisor: Steven Davis
Project Title: "Isomerization Reactions of Strained Tricyclo-Species"
Grant: NSF CHE-1460568
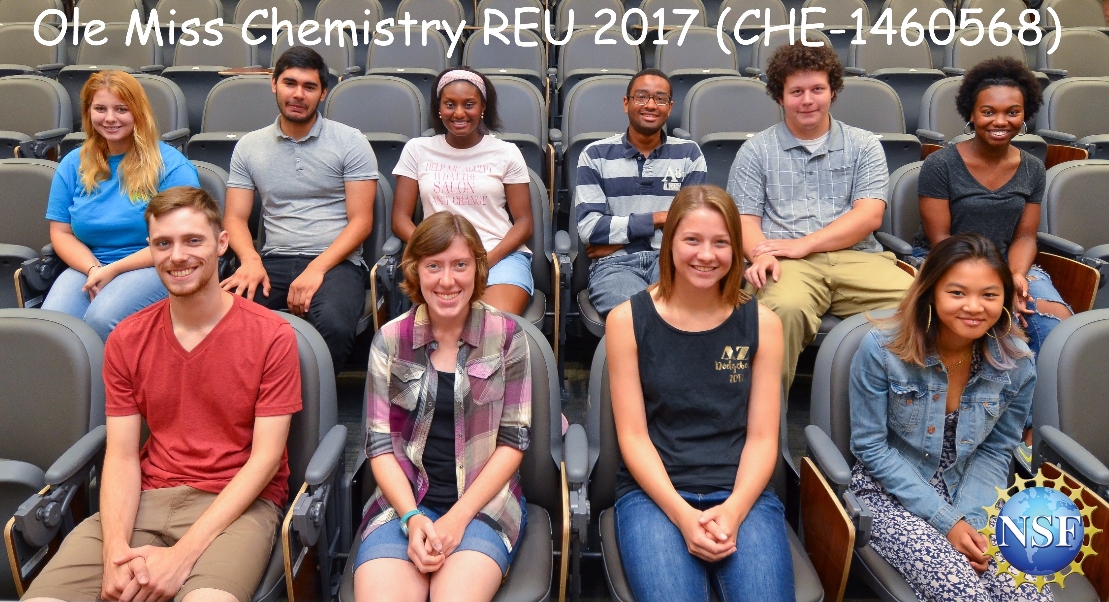
REU 2017 Students

Kamesha Adams
Home College:LeMoyne-Owen College
Faculty Advisor:Robert Doerksen
Project Title:Computational Chemistry: Analyzing New Molecules
Grant:NSF CHE-1460568

Mattie Braselton
Home College: Georgia Southern University
Faculty Advisor:Jard Delcamp
Project Title:Novel Chromophores for Dye-Sensitized Solar Cells
Grant:NSF CHE-1460568

Colleen Chernowsky
Home College:Ohio Wesleyan University
Faculty Advisor:Jonah Jurss
Project Title:Catalytic CO2 Reduction by NHC Metal Complexes
Grant: NSF CHE-1460568

Angela Dam
Home College:Temple University
Faculty Advisor:Steve Davis
Project Title:Disrotatory and Conrotatory Pathways
Grant: NSF CHE-1460568

Sydney McDonald
Home College:University of Central Arkansas
Faculty Advisor:Randy Wadkins
Project Title:Protein Purification using Affinity Chromatography
Grant: NSF CHE-1460568

Jorge Nevarez
Home College:Northern Illinois University
Faculty Advisor:Davita Watkins
Project Title:Halogen bonding in material science
Grant:NSF CHE-1460568

Cyrus Picou, Jr.
Home College:Nicholls State University
Faculty Advisor:Nathan Hammer
Project Title:Photophysical Characterization of Newly-Developed Emissive Materials
Grant:NSF CHE-1460568

Zachary Sabata
Home College:University of Nebraska at Omaha
Faculty Advisor:Kate Dooley
Project Title:Characterizing Power Losses in a Nearly Lossless Brewster's Angle Polarizing Beam Splitter
Grant: NSF CHE-1460568

Morgan Webb
Home College:Lyon College
Faculty Advisor:Gregory Tschumper
Project Title:Conformational Analysis of Furan and Thiophene Systems
Grant:NSF CHE-1460568

Chad Williams
Home College:Central Alabama Community College
Faculty Advisor:Gregory Tschumper
Project Title:Computational Analysis of 1-Ethyl-3-methylimidazolium Thiocyanate
Grant:NSF IIA-1430364

Garrett Williams
Home College:Baylor University
Faculty Advisor:Susan Pedigo
Project Title:Cadmium-Induced Dimer Disassembly of Neural Cadherin
Grant:NSF CHE-1460568
External REU 2018 Students
Virginia Baker
Home College:Delta State University
Faculty Advisor:Nathan Hammer
Project Title:A Computational and Spectroscopic Study of Cu(II)Imidazole2Cl2
Grant: NIH P20 GM103476

Brenna Bierman
Home College:Sewanee
Faculty Advisor:Jared Delcamp
Project Title:Solar Cell Dye Synthesis
Grant: NSF CHE-1460568

Dr. Jeremy Carr
Home College:Central Alabama Community College
Faculty Advisor:Greg Tschumper
Project Title:Research Opportunities in Atypical Settings: Combating Everyday Problems Using Your Chemistry Training
Grant: NSF CHE-1664998

Mary Connell
Home College:University of the Cumberlands
Faculty Advisor:Jason Ritchie
Project Title:Synthesis of a Quaternary Amine Polymer for Use in Battery Electrolytes
Grant: NSF CHE-1460568

Arthur Harris
Home College:Central Arkansas
Faculty Advisor:Davita Watkins
Project Title:It’s Synthesis of Nitrogen Containing Building Blocks as Organic Semiconducting Materials
Grant: NSF CHE-1460568

Hannah Kline
Home College:Western Carolina University
Faculty Advisor:Jonah Jurss
Project Title:Synthesis and Electrochemical Analysis of Cyclometalated Ruthenium Terpyridine-Based Catalysts for Carbon Dioxide Reduction
Grant: NSF CHE-1460568
Kiara Lugo
Home College:Georgia State University
Faculty Advisor:Saumen Chakraborty
Project Title:Synthesis, Characterization and Applications of Protein-Templated Ultrasmall Gold Nanoclusters
Grant: NSF CHE-1460568

Erica Mitchell
Home College:Taylor University
Faculty Advisor:Steven Davis
Project Title:Tracing the Theoretical Reaction Path of Benzvalyne
Grant: NSF CHE-1460568

Jacquelyn Mosely
Home College:Henderson State University
Faculty Advisor:Gregory Tschumper
Project Title:Interrogating the Vibrational C-I Stretch in a Series of Azabenzene Halogen Bond Acceptors
Grant: NSF CHE-1664998

Zachary Palmer
Home College:Georgia Southern University
Faculty Advisor:Gregory Tschumper
Project Title:Computational Exploration of Photocatalytic CO2 Reduction in Pyridyl-NHC-Ligated Rhenium
Grant: NSF OIA-1430364

William VanBenschoten
Home College:Winona State University
Faculty Advisor:Gregory Tschumper
Project Title:Dissociation Energy of the HCN-HF Dimer
Grant: NSF CHE-1460568
Noelle Watson
Home College:University of North Florida
Faculty Advisor:Nathan Hammer
Project Title:Spectroscopic and Computational Studies of Chromium(III)-Imidazole Complexes
Grant: NSF CHE-1460568

Colin Welsh
Home College:Rhodes College
Faculty Advisor:Robert Doerksen
Project Title:Computational Methods for Structural Elucidation
Grant: NSF CHE-1460568

Brent Westbrook
Home College:St. Edward's University
Faculty Advisor:Ryan Fortenberry
Project Title:Water/A+ and Hydrogen Sulfide/+ Cations (IN SPACE!!)
Grant: NSF CHE-1460568

Garrett Williams
Home College:Baylor University
Faculty Advisor:Susan Pedigo
Project Title:Cadmium-Induced Dimer Disassembly of Neural Cadherin
Grant: NSF CHE-1460568
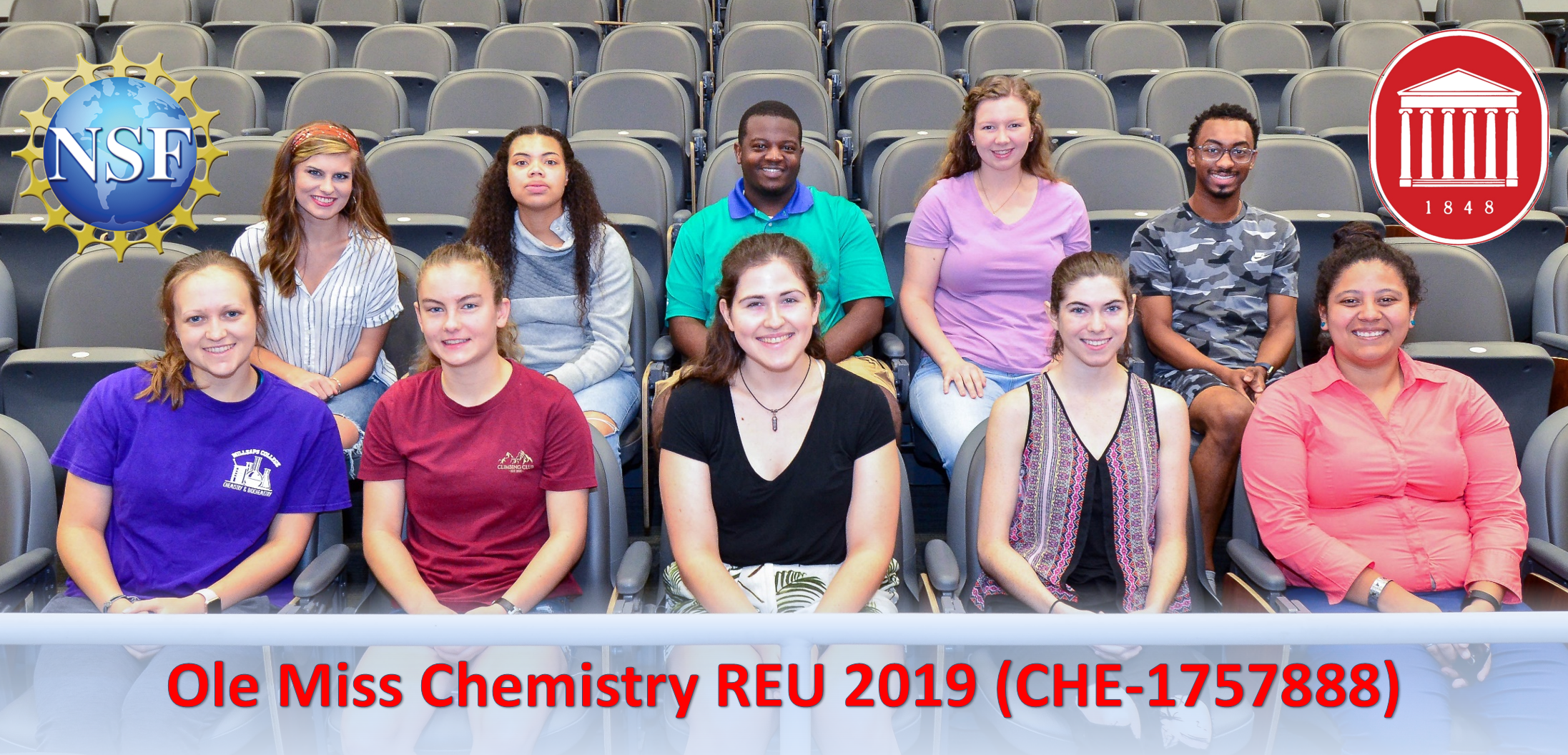
External REU 2019 Students

Summer Basham
Home College: Union University
Faculty Advisor: Jason Ritchie
Project Title: Polymer Electrolyte Membrane Synthesis
Grant: NSF CHE-1757888

Perry Broom
Home College: Mississippi College
Faculty Advisor: Gregory Tschumper
Project Title: Exploring the Potential Energy Surface of the Water-Azide Complex
Grant: NSF OIA-1757220

MaTais Caldwell
Home College: Central Alabama
Faculty Advisor: Gregory Tschumper
Project Title: Investigation of Intramolecular Hydrogen Bonding in Cyclopropanols
Grant: NSF OIA-1757220

Jamie Chamberlin
Home College: East Carolina
Faculty Advisor: Jason Ritchie
Project Title: Toward understanding Acid Dissociation in Polymer Electrolyte Medium
Grant: NASA NNX15AH78H

Ava Chard
Home College: Northern Arizona
Faculty Advisor: Steve Davis
Project Title: Formation Pathways of Elementary Magnesium PseudoPAH Molecules
Grant: NSF CHE-1757888

Athena Flint
Home College: Yale University
Faculty Advisor: Ryan Fortenberry
Project Title: Studying HeH+ Post-Detection: From Computational Methods to Spectroscopic Research
Grant: NSF CHE-1757888

Alison Fullilove
Home College: Delta State University
Faculty Advisor: Nathan Hammer
Project Title: Investigating the Formation of Hydrogen Bonds in Gamma-Amimobutryic Acid
Grant: NIH P20 GM103476

Stephen Goodlett
Home College: University of Kentucky
Faculty Advisor: Gregory Tschumper
Project Title: Anharmonic Computations of Carbonyl Sulfide Vibrations
Grant: NSF OIA-1757220

Katelyn Groenhout
Home College: Georgia Tech
Faculty Advisor: Jared Delcamp
Project Title: Solar Cells for a Brighter Future: Thienopyrazines as a π-spacer in Dye Sensitized Solar Cells
Grant: NSF CHE-1757888

Cornell Jones
Home College: Arkansas - Pine Bluff
Faculty Advisor: Nathan Hammer
Project Title: A Raman Spectroscopic and Computational Investigation of Phenoxyethanol
Grant: NSF CHE-1757888

Sarai Jaime
Home College: Bakersfield College
Faculty Advisor: Randy Wadkins
Project Title: Small Molecule Interactions in Crowded Molecular Environments
Grant: NSF CHE-1757888

Charles Zachary Palmer
Home College: Georgia Southern
Faculty Advisor: Ryan Fortenberry
Project Title: Thioformaldehyde Isomers in Space
Grant: UM College of Liberal Arts

Cassidy Soard
Home College: Missouri State
Faculty Advisor: Davita Watkins
Project Title: Creating Donor-Acceptor Polymers via Electropolymerization
Grant: NSF CHE-1757888

Anthony Sumlin
Home College: Arkansas State
Faculty Advisor: Robert Doerksen
Project Title: SCG-15: Determination of Absolute Configuration via Computational Methods
Grant: NSF CHE-1757888

Cameron Trussell
Home College: Millsaps College
Faculty Advisor: Jonah Jurss
Project Title: Development of Rhenium Catalysts for CO2 Reduction Using the Second Coordination Sphere
Grant: NSF CHE-1757888

Angel Weather
Home College: Augusta University
Faculty Advisor: Davita Watkins
Project Title: The Synthesis of Aryl Substituted Caprolactone for Janus Dendrimers
Grant: NSF CHE-1757888
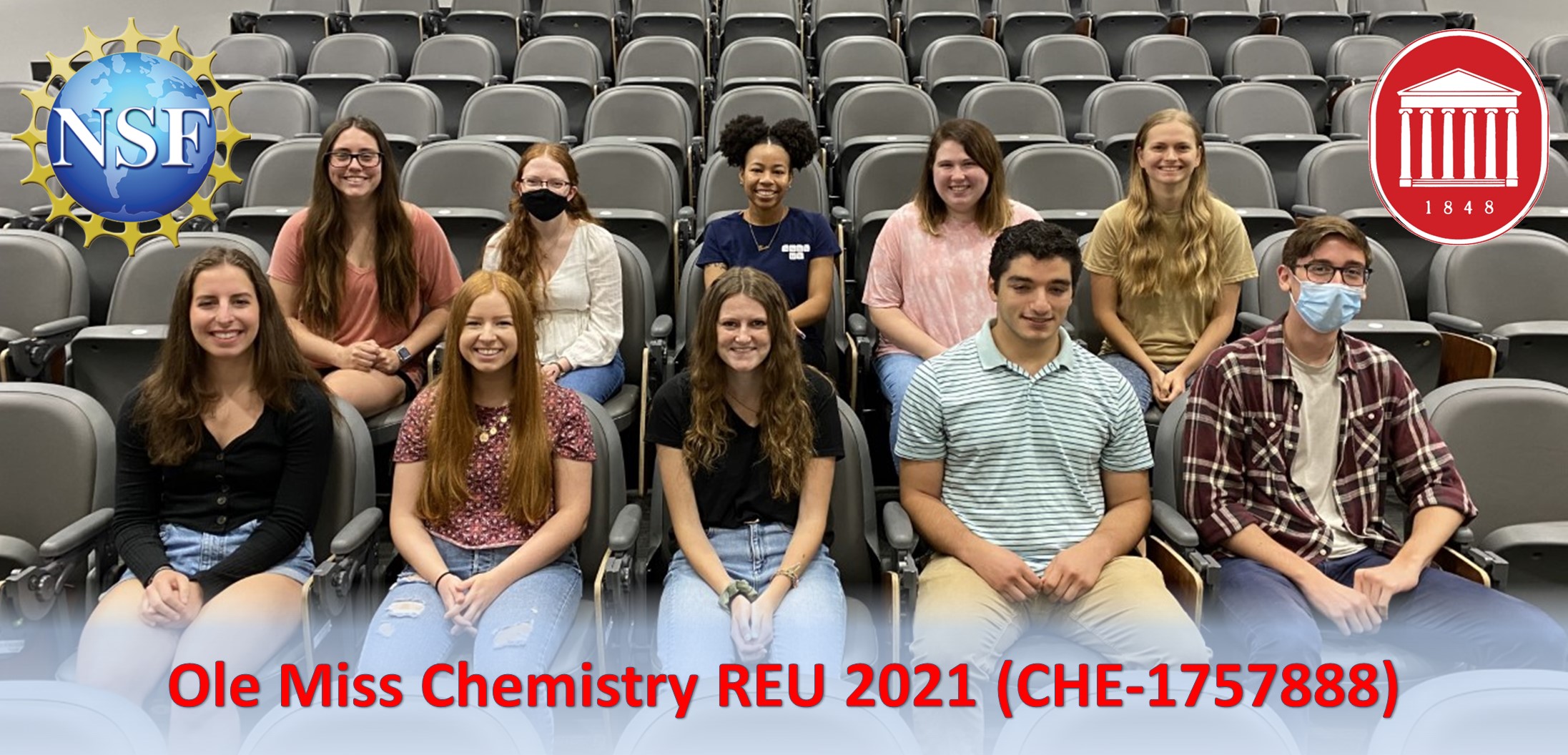
2021 REU Students

Micai Benford
Home College: Centre College
Faculty Advisor: Gregory Tschumper
Project Title: The Many Bodies of Water
Funding: NSF CHE-1664998

Mikhayla Clothier
Home College: Erskine College
Faculty Advisor: Gregory Tschumper
Project Title: Influence of an Ar Atom on the Structures, Energetics and Vibrational Frequencies of the Water Hexamer
Funding: NSF CHE-1757888

Camryn Gloor
Home College: Texas Christian University
Faculty Advisor: Jason Ritchie
Project Title: The Synthesis and Analysis of Ion Conducting Polymers
Funding: NSF CHE-1757888

Daniel Grosselin
Home College: John Brown University
Faculty Advisor: Ryan Fortenberry
Project Title: Interstellar Reaction Pathway for Magnesium Oxides and Water
Funding: NSF OIA-1757220

Lara Kockaya
Home College: Henderson State
Faculty Advisor: Robert Doerksen
Project Title: Elucidation of Daphnegiratriprenylone A Regioisomer
Funding: NSF CHE-1757888

Ainsley LaMore
Home College: Truman State University
Faculty Advisor: Jared Delcamp
Project Title: Controlling Dye Design to Promote a Long Lived Cationic State
Funding: NSF CHE-1757888

Jorden Marzette
Home College: Xavier University
Faculty Advisor: Eden Tanner
Project Title: Diffusion of Nanoparticles in Mucus for Nasal Drug Delivery
Funding: NSF CHE-1757888

Madison McGuire
Home College: Belhaven University
Faculty Advisor: Jared Delcamp
Project Title: Cross-Conjugated NIR Water Soluble Indolizine Squaraines for Fluorescence Imaging
Funding: NSF OIA-1757220

Karlee McKinney
Home College: Belhaven University
Faculty Advisor: Davita Watkins
Project Title: Developing NIR-II Small Molecule Fluorophores with Aggregate Induced Emission for Bioimaging
Funding: NSF CHE-1757888

Paul Polzer
Home College: Franciscan University
Faculty Advisor: Saumen Chakraborty
Project Title: Redesigning the Ferredoxin Scaffold into an Artificial ACS Active Site
Funding: NSF CHE-1757888

Samantha Schwartz
Home College: Mississippi College
Faculty Advisor: Jared Delcamp
Project Title: Pyrene-Based Donor Dyes for Studying Pi-Stacking in Dye-Sensitized Solar Cells
Funding: NSF OIA-1757220

Marie Strauss
Home College: Biola University
Faculty Advisor: Ryan Fortenberry
Project Title: Space Anions
Funding: NSF CHE-1757888

Morgan Ward
Home College: Saint Louis University
Faculty Advisor: Jonah Jurss
Project Title: Novel Metal Complexes for Solar Energy Production
Funding: NSF CHE-1757888

Ali Younis
Home College: Concordia College
Faculty Advisor: Randy Wadkins
Project Title: Stability of I-Motif DNA: Effects of Group 2 Cations
Funding: NSF CHE-1757888

Claudia Chambliss
Home College: University of Mississippi
Faculty Advisor: Nathan Hammer
Project Title:
Funding: NIH GM103476

Ashton Custer
Home College: University of Mississippi
Faculty Advisor: Robert Doerksen
Project Title:
Funding: NIH GM130460

Jack Flanders
Home College: University of Mississippi
Faculty Advisor: Eden Tanner
Project Title:
Funding: College of Liberal Arts

Abigail Hartline
Home College: University of Mississippi
Faculty Advisor: Eden Tanner
Project Title: Ionic Liquids for Aqueous Stabilization of Near Infrared Dye Materials
Funding: NSF OIA-1757220

Anh Hoang
Home College: University of Mississippi
Faculty Advisor: Eden Tanner
Project Title:
Funding: College of Liberal Arts

Caroline Hodge
Home College: University of Mississippi
Faculty Advisor: Jonah Jurss
Project Title:
Funding: ACS PRF 58707-DNI3

Nicholas Kruse
Home College: University of Mississippi
Faculty Advisor: Nathan Hammer
Project Title:
Funding: NSF OIA-1757220

Ethan Lambert
Home College: University of Mississippi
Faculty Advisor: Nathan Hammer
Project Title:
Funding: NSF OIA-1757220

Allison Mahurin
Home College: University of Mississippi
Faculty Advisor: Eden Tanner
Project Title:
Funding: College of Liberal Arts

Joh'nis Randall
Home College: University of Mississippi
Faculty Advisor: Eden Tanner
Project Title:
Funding: College of Liberal Arts

Austin Wallace
Home College: University of Mississippi
Faculty Advisor: Ryan Fortenberry
Project Title:
Funding: College of Liberal Arts

Alexandria Watrous
Home College: University of Mississippi
Faculty Advisor: R
Funding: College of Liberal Arts
External REU 2022 Students

Landon Ashley
Home College: Mississippi College
Faculty Advisor: Saumen Chakraborty
Project Title: Kinetics of ET in Biomolecular Copper Catalysts with Relevance to Small Molecule Activation
Funding: NSF CHE-1757888

Mikala Blackmon
Home College: University of Tennessee Knoxville
Faculty Advisor: Jason Ritchie
Project Title: Synthesis of MePEG7 Copolymer as a Conducting Electrolyte
Funding: NSF CHE-1757888
Mia Bogan
Home College: Alcorn State University
Faculty Advisor: Eden Tanner
Project Title: Drug Permeation studies dealing with Porcine oral mucosa
Funding: PhRMA Foundation

Nicholas Covalsen
Home College: Jacksonville State University
Faculty Advisor: Nathan Hammer
Project Title: Ultrafast dynamics of ionic liquid-squaraine dye systems
Funding: NSF CHE-1757888

Rebecca Firth
Home College: Tennessee Tech
Faculty Advisor: Ryan Fortenberry
Project Title: Building a planet: Al LEGOs?
Funding: NSF CHE-1757888

Martin Flores
Home College: Auburn University
Faculty Advisor: Greg Tschumper
Project Title: Intramolecular Hydrogen Bonding in Adamantane Systems
Funding: NSF CHE-1757888

Olivia Haney
Home College: Belhaven University
Faculty Advisor: Ryan Fortenberry
Project Title: Carbonic Acid: Uncovering the Ice-Cold Mystery
Funding: College of Liberal Arts

McKyan James
Home College: Rhodes College
Faculty Advisor: Eden Tanner
Project Title: Biomedical Applications of Ionic Liquids
Funding: PhRMA Foundations

Adora Norman
Home College: Alcorn State University
Faculty Advisor: Eden Tanner
Project Title: Assessing the permeation of choline and amino acid based ionic liquids on porcine skin
Funding: NSF CHE-1757888

Allison Rhoads
Home College: University of Alabama
Faculty Advisor: Randy Wadkins
Project Title: Dextran as a Crowding Agent in the Study of i-Motif Formation
Funding: NSF CHE-1757888

Samantha Schwartz
Home College: Mississippi College
Faculty Advisor: Jared Delcamp
Project Title: Pyrene-Based Donor Dyes for Studying Pi-Stacking in Dye-Sensitized Solar Cells
Funding: NSF CHE-1757888

Christopher Sehring
Home College: Delta State University
Faculty Advisor: Ryan Fortenberry
Project Title: Dust in the Interstellar Wind
Funding: NASA 80NSSC20K0001

Zachary Tomlinson
Home College: Tiffin University
Faculty Advisor: Jonah Jurss
Project Title: Synthesis of Metal Catalysts for Water Oxidation
Funding: NSF CHE-1757888

Max Tucker
Home College: Washington College
Faculty Advisor: Robert Doerksen
Project Title: Computational methods for small-molecule drug discovery
Funding: NSF CHE-1757888
2022 Internal UM REU Students

Christopher Austin
Home College: University of Mississippi
Faculty Advisor: Jonah Jurss
Funding: NSF CHE-1848478

Matthew Bee
Home College: University of Mississippi
Faculty Advisor: Jim Cizdziel
Project Title: Microplastics in Urban Stormwater Runoff
Funding: Hearin Foundation

Alex Bromley
Home College: University of Mississippi
Faculty Advisor: Jonah Jurss
Funding: NSF OIA-1757220

Carly Clisham
Home College: University of Mississippi
Faculty Advisor: Jim Cizdziel
Project Title: Forensic analysis of automobile paint layering by FTIR microscopy
Funding: Hearin Foundation

Jon Dotson
Home College: University of Mississippi
Faculty Advisor: Ryan Fortenberry
Funding: College of Liberal Arts

Meghan Gorniak
Home College: University of Mississippi
Faculty Advisor: Eden Tanner
Funding: PhRMA Foundation

Nicole Guerin
Home College: University of Mississippi
Faculty Advisor: Eden Tanner
Funding: College of Liberal Arts

Bekah Heintz
Home College: University of Mississippi
Faculty Advisor: Eden Tanner
Project Title: Permeation of choline-based ionic liquid nanoparticles through porcine nasal mucosa
Funding: PhRMA Foundation

Anh Hoang
Home College: University of Mississippi
Faculty Advisor: Eden Tanner
Funding: PhRMA Foundation

Deauntaye Jones
Home College: University of Mississippi
Faculty Advisor: Eden Tanner
Funding: PhRMA Foundation

Nicholas Kruse
Home College: University of Mississippi
Faculty Advisor: Nathan Hammer
Funding: NSF OIA-1757220

Ethan Lambert
Home College: University of Mississippi
Faculty Advisor: Nathan Hammer
Project Title: Probing halogen bonding interactions between heptafluoro-2-iodopropane and three azabenzenes with Raman spectroscopy and density functional theory
Funding: NSF OIA-1757220

Timothy Lewis
Home College: University of Mississippi
Faculty Advisor: Jared Delcamp
Project Title: Synthesis and Photophysical Properties of SiRosindolizine Derivatives for SWIR Fluorescence Imaging
Funding: NSF OIA-1757220

Ivy Li
Home College: University of Mississippi
Faculty Advisor: Jared Delcamp
Funding: DE-SC0019131
George Monroe
Home College: University of Mississippi
Faculty Advisor: Eden Tanner
Project Title: Targeted drug delivery via ionic liquid coated nanoparticles
Funding: PhRMA Foundation

Isabel Nichols
Home College: University of Mississippi
Faculty Advisor: Eden Tanner
Funding: NSF OIA-1757220

Skylar Nichols
Home College: University of Mississippi
Faculty Advisor: Jonah Jurss
Funding: NSF CHE-1848478

Mercedes Pride
Home College: University of Mississippi
Faculty Advisor: Eden Tanner
Funding: PhRMA Foundation

Angela Roberts
Home College: University of Mississippi
Faculty Advisor: Eden Tanner
Project Title: Tunability of Nanoparticles Degradation
Funding: PhRMA Foundation

Emma Routier
Home College: University of Mississippi
Faculty Advisor: Eden Tanner
Funding: College of Liberal Arts

Madison Schroeder
Home College: University of Mississippi
Faculty Advisor: Eden Tanner
Funding: College of Liberal Arts

Ember Suh
Home College: University of Mississippi
Faculty Advisor: Eden Tanner
Funding: McNair Scholar

Brinley Tapp
Home College: University of Mississippi
Faculty Advisor: Eden Tanner
Funding: College of Liberal Arts

Mary Beth Vanlandingham
Home College: University of Mississippi
Faculty Advisor: Eden Tanner
Project Title: Diffusion of Choline-Based Ionic Liquid Coated Nanoparticles in Nasal Mucus
Funding: CPhRMA Foundation

Alexandria Watrous
Home College: University of Mississippi
Faculty Advisor: Ryan Fortenberry
Project Title: Isomers of C5H2: A Computational Study
Funding: College of Liberal Arts
External REU 2023 Students

Bryson Anderson
Home College: University of Utah
Faculty Advisor: Randy Wadkins
Project Title: The Effects of group 2 metals on the stability of i-Motif DNA
Grant: NSF CHE-2150352

Akira Clark
Home College: Northwest Mississippi
Faculty Advisor: Kensha Clark
Project Title: Towards Electron Transfer Using Synthesis of Zinc Chloride Ligand Complex for Redox Noninnocent Derivatives
Grant: NSF CHE-2150352

Sarah Clouse
Home College: Univ. North Alabama
Faculty Advisor: Jason Ritchie
Project Title: Investigating Hydrogen Attachment Sites in Polymer Electrolyte Membranes: Understanding Fuel Cell Mechanics
Grant: NSF CHE-2150352

Remy Cron
Home College: Alabama at Birmingham
Faculty Advisor: Ryan Fortenberry
Project Title: Quantum Mechanical Determination of Promising Biomedical Molecular Dyes
Grant: NSF CHE-1757888

Taylor Gregory
Home College: Western Carolina
Faculty Advisor: Jim Cizdziel
Project Title: μ-FTIR Identification of Binders in Prehistoric Rock Paintings from the Lower Pecos Archaeological Region
Grant: NSF CHE-2150352

Abigail Haskew
Home College: Oklahoma Christian
Faculty Advisor: Robert Doerksen
Project Title: Accurate Prediction of Lectin-Glycan Complexes: Exploring the Accuracy of Docking Software
Grant: NSF CHE-2150352

Garrett Hidalgo
Home College: LeTourneau University
Faculty Advisor: Jonah Jurss
Project Title: Synthesizing Ruthenium Dinuclear Catalysts for Water Oxidation With Anionic Bridging Ligands
Grant: CHE-2150352

Zori Jackson
Home College: Mississippi State
Faculty Advisor: Vignesh Sundaresan
Project Title: Building CLocK Microscopy for Imaging Electrochemical Reactions
Grant: NSF OIA-1757220

Whitney Jones
Home College: Tougaloo College
Faculty Advisor: Eden Tanner
Project Title: Targeting Endometriosis with Gold Nanoparticles and Neutrophil Hitchhiking: Changing the Way We Approach Female Reproductive Medicine
Grant: NSF CHE-2204193

Yaisa Juarez
Home College: Nevada State Univ.
Faculty Advisor: Kensha Clark
Project Title: Towards Electron Transfer Using Synthesis of Zinc Chloride Ligand Complex for Redox Noninnocent Derivatives
Grant: NSF CHE-1757888

Grace Liles
Home College: University of Missouri
Faculty Advisor: Eden Tanner
Project Title: Glyco-Ionic Liquid Coated Nanoparticles for Targeted Cancer Treatment
Grant: NSF CHE-2150352

Joshua Paul
Home College: University of Portland
Faculty Advisor: Jonah Jurss
Project Title: Synthesis of Terpyridine Ligands for the Purpose of Water Oxidation
Grant: NSF OIA-1757220

Christopher Sehring
Home College: Delta State University
Faculty Advisor: Ryan Fortenberry
Project Title: ImPAHsibbly Simulating Infrared Spectra
Grant: NSF CHE-2150352

Noella Tantine
Home College: Nevada State Univ.
Faculty Advisor: Vignesh Sundaresan
Project Title: Synthesis and characterization of ionic liquids coated Au nanoparticles
Grant: NSF CHE-2150352

Madeline Thomas
Home College: University of Alabama
Faculty Advisor: Gregory Tschumper
Project Title: Calibrating DFT methods for the hydration of superhalogens: structures, energetics and vibrational signatures
Grant: NSF CHE-2150352
External REU 2024 Students

Caleb Bellamy
Home College: North Carolina A&T
Faculty Advisor: Kensha Clark
Project Title: Hydrogenation with Constrained Geometry Catalyst
Grant: NSF CHE-2150352

Samantha Cox
Home College: Drury University
Faculty Advisor: Randy Wadkins
Project Title: Making ‘M&Ms’ from Nanodiamonds and DNA
Grant: NSF CHE-2150352

Lauren Driggers
Home College: Presbyterian College
Faculty Advisor: Eden Tanner
Project Title: Ionic-Liquid Coated Gold Core Polymeric Nanoparticles for Targeted Photothermal Treatment of Endometriosis
Grant: NSF CHE-2150352

Emma Foley
Home College: Iowa State University
Faculty Advisor: Jonah Jurss
Project Title: Synthesizing and Characterizing Phenanthroline-Based Cobalt Catalyst for CO2 Reduction
Grant: NSF OIA-1757220

Rachel Hambuchen
Home College: Skidmore College
Faculty Advisor: Jim Cizdziel
Project Title: Quantifying Microplastics in Oysters of the Mississippi Sound Through μ-FTIR Spectroscopy
Grant: NSF CHE-2150352

Lucas Lemas
Home College: University of Portland
Faculty Advisor: Penghao Li
Project Title: Carbon Changelings: Synthesis of Shape-Shifting 3D Carbon Nanoarchitectures
Grant: CHE-2150352

Julian Manila
Home College: Colorado Mesa Univ.
Faculty Advisor: Jonah Jurss
Project Title: Synthesis of terpyridine-based ligands for CO2 reduction
Grant: NSF CHE-2150352

J’ya Massey
Home College: NC A&T University
Faculty Advisor: Eden Tanner
Project Title: Enhancement of High Molecular Brightness of NIR-Dye using Bioinspired Ionic Liquids for Bioimaging Applications
Grant: NSF CHE-2204193

Eden Nickolson
Home College: Central Alabama CC
Faculty Advisor: gregory Tschumper
Project Title: Calibrating DFT Methods for Concerted Proton Transfer Reactions
Grant: NSF CHE-2154403

Xia Parkes
Home College: George Mason Univ.
Faculty Advisor: Saumen Chakraborty
Project Title: "De Novo Designed Artificial Metallopeptidase
Grant: NSF CHE-2150352

Lana Rodriguez
Home College: Northern Illinois Univ.
Faculty Advisor: Vignesh Sundaresan
Project Title: "Electrochemical Determination of the Adsorption of Heavy Metals by Microplastics with Different Shapes
Grant: NSF CHE-2150352

Phaedra Salerno
Home College: Williams College
Faculty Advisor: Gregory Tschumper
Project Title: Calibrating DFT Methods for Anion Hydration
Grant: NSF CHE-2150352

Benjamin Taylor
Home College: St. Norbert College
Faculty Advisor: Ryan Fortenberry
Project Title: Computational Exploration of Calcium Hydride and Water Reaction Profiles in the ISM
Grant: NSF CHE-2150352
Application and Key Dates
Key Dates for 2025:
02/24/2025: Applications Due03/14/2025: Notice of Invitation to Participate
05/27/2025: Experience Begins (Move-In)
08/01/2025: Experience Ends (Move-Out)

Testimonials
Hanfei Wang
My time at the Ole Miss Research Experience for Undergraduates was highly rewarding in that I got the opportunity to work on a project which interested me and was able to learn about the world of chemistry research. Even though the experience is designed for and is most helpful for those who are pursuing a graduate degree in chemistry and a career in research, being able to spend the summer working full-time under the direction of a professor and being involved in the research process is an experience that will serve a student interested in a career in science in general extremely well, regardless of what specific discipline the student wants to pursue, or whether the student intends to pursue a career in academic research at all – the skills that are learned from being involved in the research process, such as teamwork, discipline, resilience, attention to detail, critical thinking, and analysis will prove to be useful in a variety of careers that involve science. I was extremely fortunate to have this opportunity, and I would like to thank all of the professors in the Department of Chemistry at Ole Miss, especially Dr. Hammer, the director of this program, and Dr. Wadkins, my advisor who has guided me through the project that I did, for making the REU possible.
Justin Parmely
I came to Ole Miss with absolutely no chemistry research experience. Although I had greatly enjoyed my classes and laboratory activities at my parent university, we lacked many of the resources needed to conduct meaningful scientific research. I knew that applying my chemical knowledge to real world problems would be significantly more rewarding than memorizing facts from textbooks. Even then, I underestimated the impact of my summer at the University of Mississippi. My Ole Miss REU gifted me the opportunity to interact with several of the brightest minds in biochemistry and pharmacology while simultaneously surrounding me with young adults who are likewise interested in impacting their community through scientific inquiry. Moreover, I can't imagine working with a nicer group of people; the faculty and student researchers are so genuinely kind and encouraging. As an external research student, I never felt like an outsider, and I loved it. I left a little piece of my heart in Oxford, and I now have a better understanding of how exciting a career in research can be.
Valerie Huang
At the beginning of this summer, I didn't know what to expect- it was going to be my first time going to the South, and I wasn't sure how my experience would go at all, and if I would enjoy my summer at all. Now, I can safely say that I'm so glad that I participated in the Ole Miss PChem REU because the students and faculty made my first Southern experience one to remember. Not only was I able to learn a lot from the research that I did, but I was also able to make friends that I know I will keep in contact with for a while!
Alexa Zylstra
Ole Miss REU program was the highlight of my summer. Not only did I learn important technical skills in the lab, I was able to form relationships with other scientists. The program also provided fun activities for us to all bond over and have an awesome time.
Harassment Reporting
If you are the victim of harassment by other students or faculty, you should contact the appropriate authorities at the University of Mississippi (UM) and the National Science Foundation (NSF). More information for the University of Mississippi can be found by clicking here(sexual assult) or clicking here(Title IX). Click here for information regarding Sexual Harassment from the National Science Foundation.
UM Contact Information: titleix@olemiss.edu or 662-915-7045
NSF Contact Information: programcomplaints@nsf.gov or 703-292-8020
 Click Here to Submit an Anonymous Comment, Suggestion, or Report
Click Here to Submit an Anonymous Comment, Suggestion, or Report Click Here for the UMSAFE website
Click Here for the UMSAFE website
Click here for information regarding Sexual Harassment from the National Science Foundation
Welcome to Summer Chemistry Research at the University of Mississippi

The Ole Miss Physical Chemistry Summer Research Program is supported by an NSF Research Experiences for Undergraduates (REU) site (CHE-1156713, CHE-1460568, CHE-1757888, and CHE-2150352), the NSF Experimental Program to Stimulate Competitive Research (EPSCoR), including EPSCoR Track 2 (OIA-1539035) and Track 1 (EPS-0132618, EPS-0903787, and OIA-1757220) awards, and single investigator awards, including NSF CHE-0955550, CHE-0957317, CHE-1455167, CHE-1664998, CHE-1954922 and various NASA and NIH awards. The goals of the program are to:
1. Offer directed research opportunities during the summer to undergraduate students.
2. Provide training in the form of lectures and mini-courses from the faculty.
3. Offer opportunities for students to
learn how design, synthesis, and characterization work together.
4. Allow students (high school, undergraduate, and graduate) to present research talks (20 to 40 min) to a large (50+) peer audience.
5. Develop a student cohort through social activities to help promote
chemistry as a viable career option for undergraduate students.
For more information, click on "NSF REU Site" on the Menu above. To apply for the University of Mississippi NSF REU program, click the link below.


#NSFfunded #NSFREU
2025 Calendar
Participation in Group Activities Acknowledges Consent to Report the Results of Group Activities Including the Use of Likeness, Image, Voice, and/or Appearance as Such May be Embodied in any Photos, Video Recordings, Audiotapes, Digital Images, and the Like, Taken or Made on Behalf of the Summer Program or its Partners.
| Monday | Tuesday | Wednesday | Thursday | Friday |
|---|---|---|---|---|
| May 26 Memorial Day |
27 REU Students Arrive Check into Residence Hall, Pick up ID, Get Parking Pass | 28 Meet in Coulter Hall Room 211 at 9:50am Appropriate Behavior Training in Coulter 200 at 10:00am Social Activity |
29 Lecture by Prof. Ryan Fortenberry Coulter Hall 200 10:00am "Literature Searching Using the UM online Library" Social Activity Chemistry Yoga, Coulter 422 11:30am - 1:00pm |
30 Lecture by Prof. Ryan Fortenberry Coulter Hall 200 10:00am "Astrochemistry" |
| June 2 Lecture by Dr. Athena Flint Coulter Hall 200 10:00am "An Introduction to Computational Chemistry" |
3 Lecture by Prof. Vignesh Sundaresan Coulter Hall 200 10:00am "Leveraging Vikings’ Navigation Technique for Single Nanoparticle Studies" |
4 Lecture by Prof. Dan Mattern Coulter Hall 200 10:00am "A Brief History of Scientific Misconduct" Group Picture Coulter 211 11:00am |
5 Lecture by Prof. Penghao Li Coulter Hall 200 10:00am "The art of molecular carbon nanoarchitectures" Social Activity Chemistry Yoga, Coulter 422 11:30am - 1:00pm |
6 Lecture by Dr. C. Zachary Palmer Coulter Hall 200 10:00am "Applications of Computational Chemistry to Astrochemistry" |
| 9 |
10 Lecture by Prof. Eden Tanner Coulter Hall 200 10:00am "Using Physical Chemistry to Unlock Secrets in Drug Delivery" |
11 Lecture by Prof. Nathan Hammer Coulter Hall 200 10:00am "An Introduction to Spectroscopy" |
12 Lecture by Dr. Claylee Chism Coulter Hall 200 10:00am "Combating Antibiotic Resistance in Septic Infections with Choline-Carboxylate Ionic Liquid Materials as Bacterial Capture Devices" Social Activity Chemistry Yoga, Coulter 422 11:30am - 1:00pm | 13 Lecture by Prof. Sujay Ray Coulter Hall 200 10:00am "Lighting Up Life: From Glowing Dyes to Shining Proteins" |
| 16 Lecture by Priyavrat Vashisth (Tanner Group) Coulter Hall 200 10:00am "Smart Nanoparticles for Endometriosis: Harnessing Neutrophils and Heat for Targeted Therapy" |
17 Lecture by Prof. Jinchou Lou Coulter Hall 200 10:00am "Responsive Materials and Chemical Tools for Probing Biological Systems" |
18 Lecture by Prof. Jonah Jurss Coulter Hall 200 10:00am "Artificial Photosynthesis: Catalysts for Solar-to-Fuel Conversion Chemistry" |
19 Lecture by Prof. Abby Boyd Coulter Hall 200 10:00am "Topological data analysis as an alternative to machine learning in chemistry" Social Activity Chemistry Yoga, Coulter 422 11:30am - 1:00pm |
20 Lecture by Prof. Ryan Fortenberry Coulter Hall 200 10:00am "Scientists Should Write Like Journalists and Talk Like Cavemen" |
| 23 |
24 Lecture by Prof. Joshua Sharp Coulter Hall 200 10:00am "Probing Protein-Carbohydrate Interactions Using Mass Spectrometry" |
25 Lecture by Prof. Jason Ritchie Coulter Hall 211 10:00am "Enabling the Hydrogen Economy: New Electrolyte Materials in Fuel Cells and Batteries" |
26 Masters Thesis Seminar by Margaret Stucky (Hammer Group) Coulter Hall 211 10:00am "Halogen and hydrogen bonding interactions with 2,6-dimethoxypyridine" Social Activity Coulter Hall Lobby 11:00am LGBTea |
27 |
| 30
|
July 1 Lecture by Prof. Jim Cizdziel Coulter Hall 211 10:00am "Micro- and Nano-Plastic Pollution: Small Particles, Big Problems?" Reb Researcher Presentations Coulter Hall 211 1:00pm |
2 Lecture by Prof. Steven Davis Coulter Hall 211 11:00am "Energy: Past, Present, and Future" |
3 Social Activity Coulter Hall Lobby 11:00am Chemistry Basketball | 4
Fourth of July Fireworks 9:00pm mTrade Park, Hwy 314 |
| 7 |
8 Lecture by Katy Howie (Chakraborty Group) Coulter Hall 211 10:00am "Protein Engineering Approaches to Harvest Solar Energy, Catalytic C-H Activation and Battle Cancer" Social Activity Coulter Hall Lobby 11:00am Chemistry Volleyball |
9 Personal Statement Workshop Coulter Hall 211 10:00am |
10 Lecture by Prof. Kensha Clark Coulter Hall 211 10:00am "A Hop, a Skip, and a Jump: Enabling Fast Electron Transfer with Redox Active Ligands" Social Activity TBA |
11 Lecture by Prof. Saumen Chakraborty Coulter Hall 211 10:00am "At the Crossroads of Metals and Biology"
|
| 14 |
15 Lecture by Prof. Nikki Reinemann Coulter Hall 211 10:00am "Motor Proteins: Molecular Drivers of Life and Disease"
|
16 Lecture by Prof. Randy Wadkins Coulter Hall 211 10:00am "Biophysics of DNA" Grove Scholars Outreach 2:00pm |
17 Lecture by Prof. Robert Doerksen Coulter Hall 200 10:00am "An Introduction to Computational Medicinal Chemistry" Social Activity |
18 |
| 21 |
22 Overview of Grad School and Graduate Student Panel Coulter Hall 211 10:00am |
23 INBRE/EPSCOR Conference If Your Mentor is Participating |
24 INBRE/EPSCOR Conference If Your Mentor is Participating Social Activity Chemistry Yoga, Coulter 422 11:30am - 1:00pm |
25 Student Presentations Lecture by Megan McKissick Lecture by Elaina Heath Lecture by Brandon Suh |
| 28 Student Presentations Lecture by Tauheedah Brady Lecture by Maggie Simmons Lecture by Rita Zhou-Wang Lecture by Michael Harms Lecture by Briana Gamboa |
29 Student Presentations Lecture by Reagan Nichols Lecture by Tyreeanna Thompson Lecture by Camille Kuntz Lecture by Addison Smith Lecture by Drew Martin Lecture by Blake Williamson Lecture by Charlie Earl End of Summer Collaboration Workshop |
30 Student Presentations Lecture by Mira Patel Lecture by Diego A. Martínez Sebastian Lecture by Jakob Liggons Lecture by Isaac Stiles |
31 Student Presentations Lecture by Nathan Moll Lecture by John Cooley Lecture by Lynn Nguyen |
August 1 REU ENDS STUDENTS TRAVEL HOME |
REU Publications
C. M. Sehring, J. A. Johns, V. J. Esposito, and R. C. Fortenberry, “Reparameterized Semiempirical Anharmonic IR Spectra of Neutral PAHs: Benchmarking and Predictions for PAHs with More than Five Rings,” The Journal of Physical Chemistry A, 129, 6623–6631 (2025). (NSF OIA-2150352)
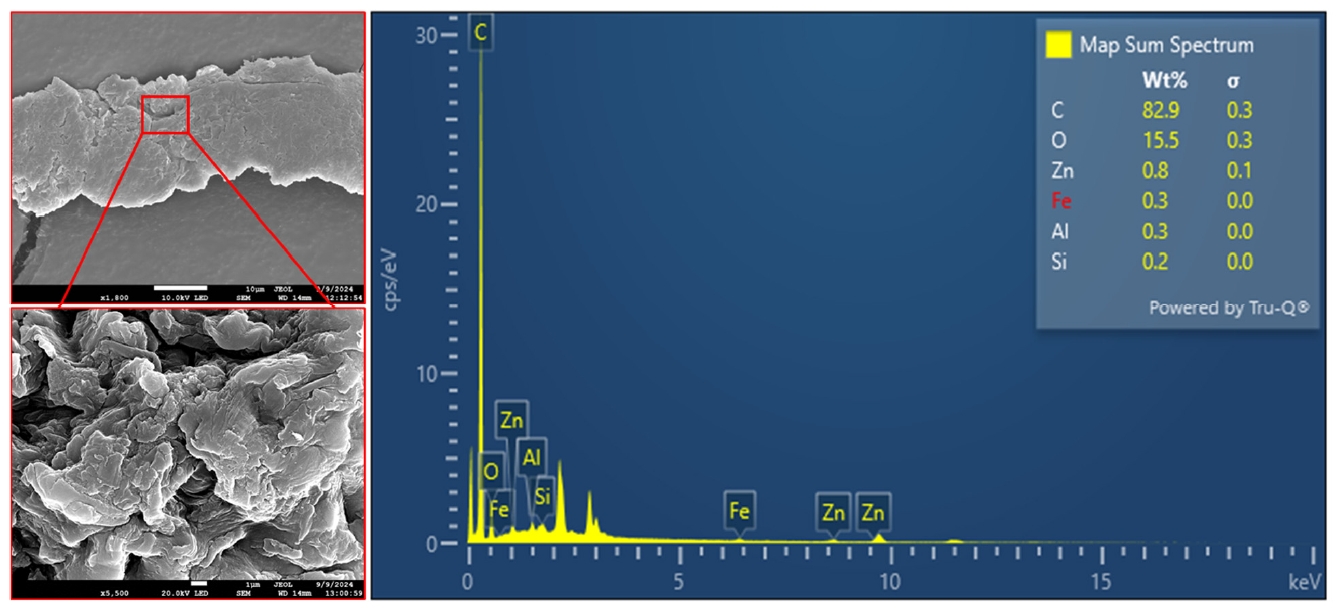
B. S. Olubusoye, J. V. Cizdziel, K. Wontor, R. Li, R. Hambuchen, V. T. Aminone, M. T. Moore, and E. R. Bennett, “Field Evaluation of Rice Husk Biochar and Pine Tree Woodchips for Removal of Tire Wear Particles from Urban Stormwater Runoff in Oxford, Mississippi (USA),” Sustainability, 17, 4080 (2025). (NSF OIA-2150352)
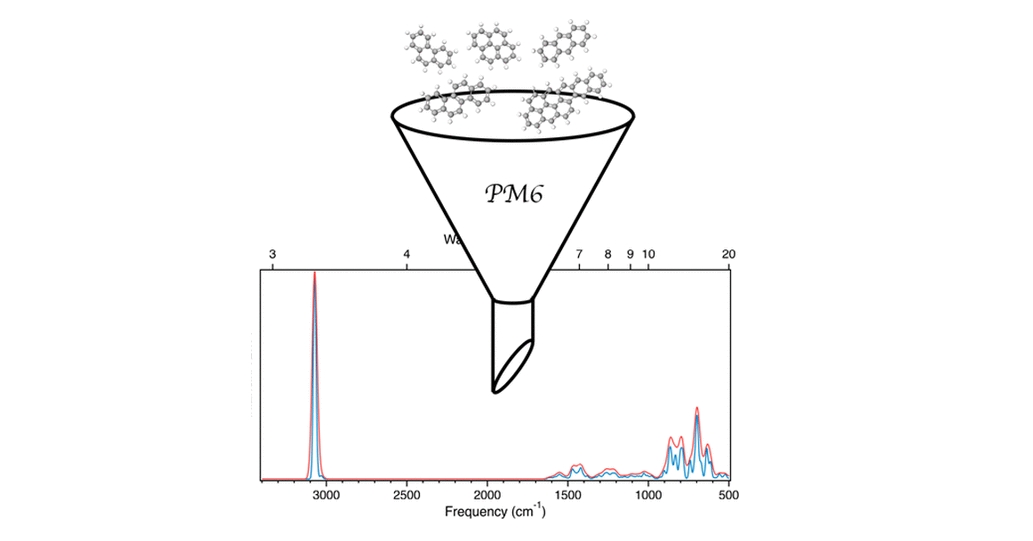
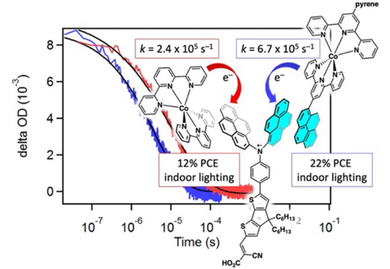
R. R. Cron, J. South, and R. C. Fortenberry, “Quantum Chemical Determination of Molecular Dye Candidates for Non-Invasive Bioimaging ,” Molecules, 29 5860 (2024). (NSF OIA-2150352)
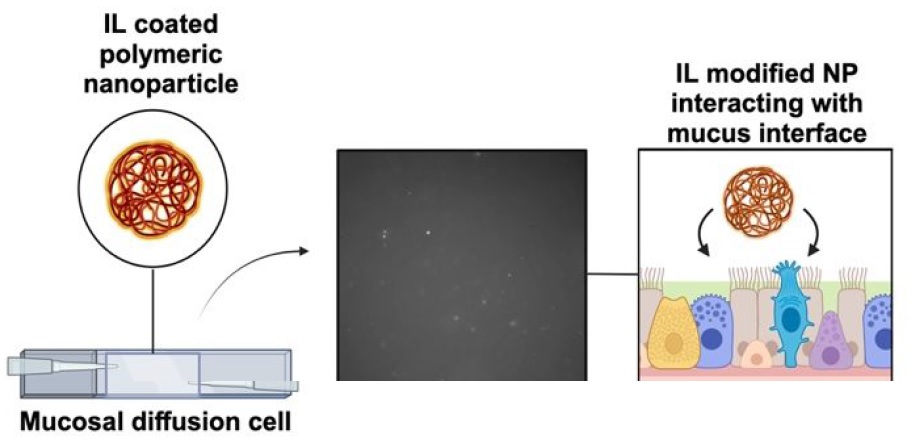
M. E. VanLandingham, R. A. Heintz, C. S. Kariyawasam, D. S. Darlington, C. M. Chism, S. X. Edgecomb, A. Roberts, J. Marzette, N. C. Fitzkee, E. E. L. Tanner, “Ionic Liquid-Modified Nanoparticles as Potential Mucus Modulators for Nasal Drug Delivery,” ACS Applied Nano Materials, 7, 18309–18317 (2024). (NSF OIA-1757888)
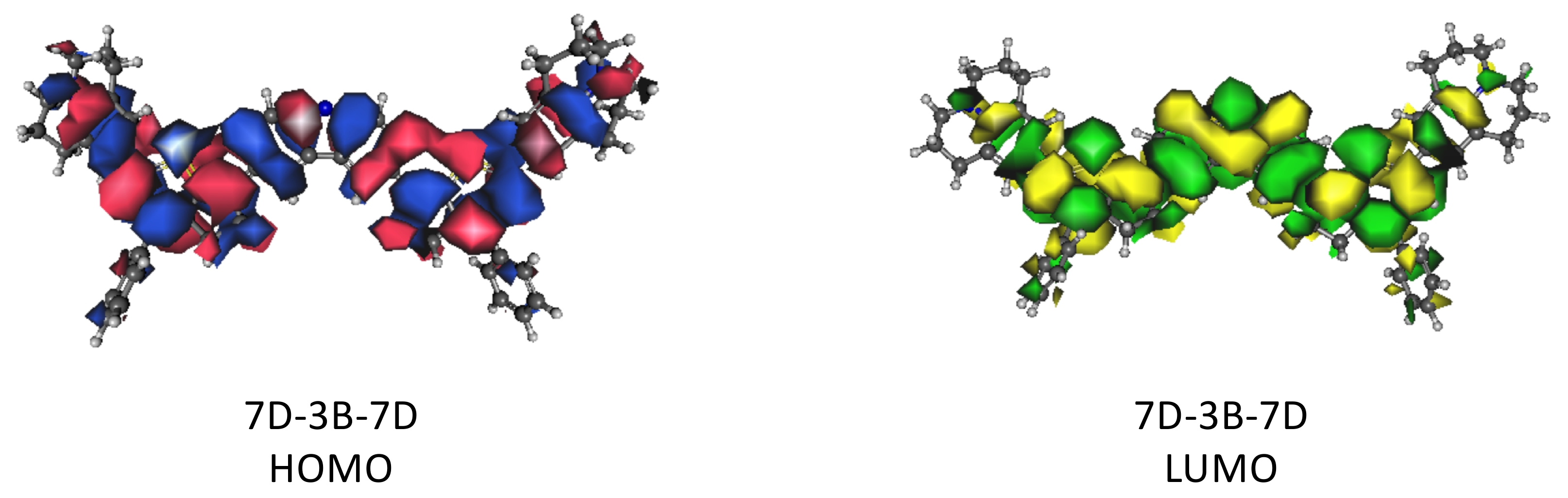
C. Curiac, E. C. Lambert, L. A. Hunt, M. Roberts, A. LaMore, A. Peddapuram, H. Cheema, N. I. Hammer, and J. H. Delcamp, “Increasing Photoinduced Interfacial Charge Separation Lifetime Through Control of Twist Angle at the Donor Region of Carbazole-Based Dyes,” Journal of Physical Chemistry C (2023). DOI: 10.1021/acs.jpcc.3c04735 (NSF OIA-1757888)
L. N. Olive, E. V. Dornshuld, H. F. Schaefer III, and G. S. Tschumper, “Competition between Solvent···Solvent and Solvent···Solute Interactions in the Microhydration of the Tetrafluoroborate Anion, BF4–(H2O)n=1,2,3,4,” Journal of Physical Chemistry A, 127, 8806–8820 (2023). DOI: 10.1021/acs.jpca.3c04014 (CHE-1757888)

W. E. Meador, N. P. Liyanage, J. Watson, K. Groenhout, and J. H. Delcamp, “Panchromatic NIR-Absorbing Sensitizers with a Thienopyrazine Auxiliary Acceptor for Dye-Sensitized Solar Cells,” Applied Energy Materials, 6, 5416–5428 (2023). DOI: 10.1021/acsaem.3c00519 (CHE-1757888)
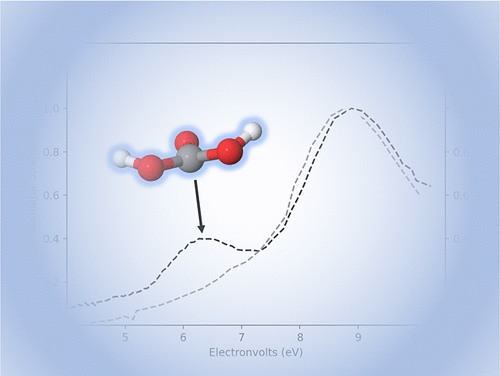
O. G. Haney, B. R. Westbrook, T. J. Santaloci, and R. C. Fortenberry, “Red-Shifting the Excitation Energy of Carbonic Acid Clusters Via Nonminimum Structures,” Journal of Physical Chemistry A, 127, 489–494 (2023). DOI: 10.1021/acs.jpca.2c07589 (CHE-1757888)
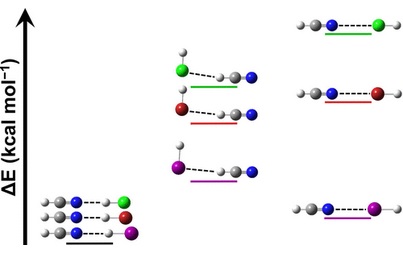
M. A. Perkins and G. S. Tschumper, “Characterization of Competing Halogen- and Hydrogen-Bonding Motifs in Simple Mixed Dimers of HCN and HX (X = F, Cl, Br, and I),” Journal of Physical Chemistry A, 126, 3688–3695 (2022). DOI: 10.1021/acs.jpca.2c02041 (CHE-1757888)
D. Nugegoda, S. Bhattacharya, L. A. Hunt, S. J. Schwartz, Z. H. Turner, N. I. Hammer, J. W. Jurss, and J. H,. Delcamp, “Designing Self-Assembled Dye–Redox Shuttle Systems via Interfacial π-Stacking in Dye-Sensitized Solar Cells for Enhanced Low Light Power Conversion,” Energy & Fuels, 36, 7075 (2022). DOI: 10.1021/acs.energyfuels.2c00633 (CHE-1757888)
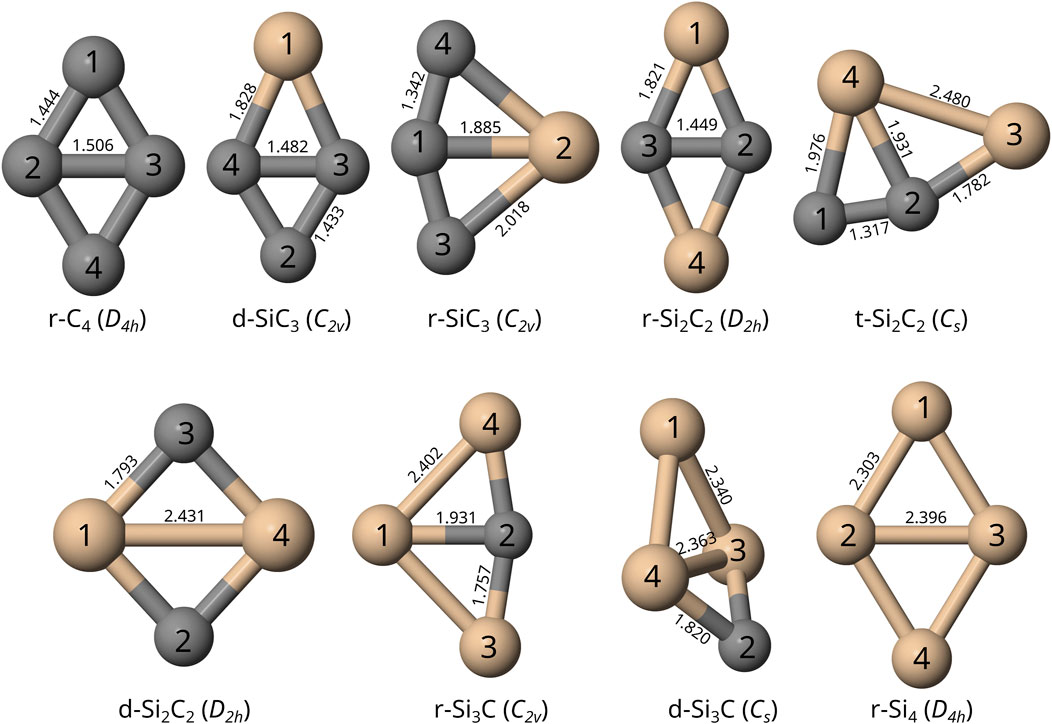
C. M. Sehring, C. Z. Palmer, B. R. Westbrook, and R. C. Fortenberry, “The spectral features and detectability of small, cyclic silicon carbide clusters,” Frontiers Astronomy and Space Sciences, 9, 1074879 (2022). DOI: 10.3389/fspas.2022.1074879 (CHE-1757888)
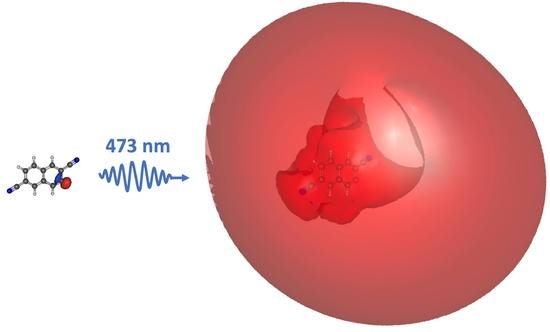
M. E. Strauss, T. J. Santaloci, and R. C. Fortenberry, “Valence-, Dipole- and Quadropole-Bound Electronically Excited States of Closed-Shell Anions Formed by Deprotonation of Cyano- and Ethynyl-Disubstituted Polycyclic Aromatic Hydrocarbons,” Chemistry, 4, 42-56 (2022). DOI: 10.3390/chemistry4010004 (CHE-1757888)
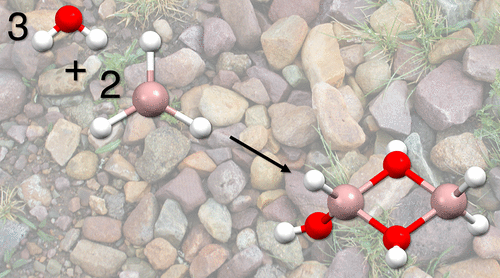
D. Grosselin and R. C. Fortenberry, “Formation of Magnesium and Aluminum Oxides from Water and Metal Hydrides: Creation of the Smallest Ruby,” ACS Earth and Space Chemistry, 6, 18-24 (2021). DOI:10.1021/acsearthspacechem.1c00324 (CHE-1757888)
T. J. Santaloci, M. E. Strauss, and R. C. Fortenberry, “Electronically Excited States of Potential Interstellar, Anionic Building Blocks for Astrobiological Nucleic Acids,” Fronteirs in Astronomoy and Space Sciences, 8, 777107 (2021). DOI:10.3389/fspas.2021.777107 (CHE-1757888)
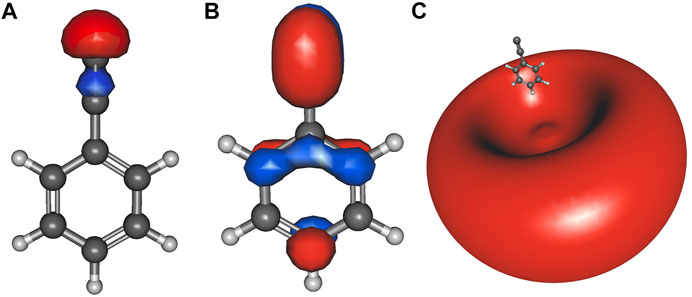
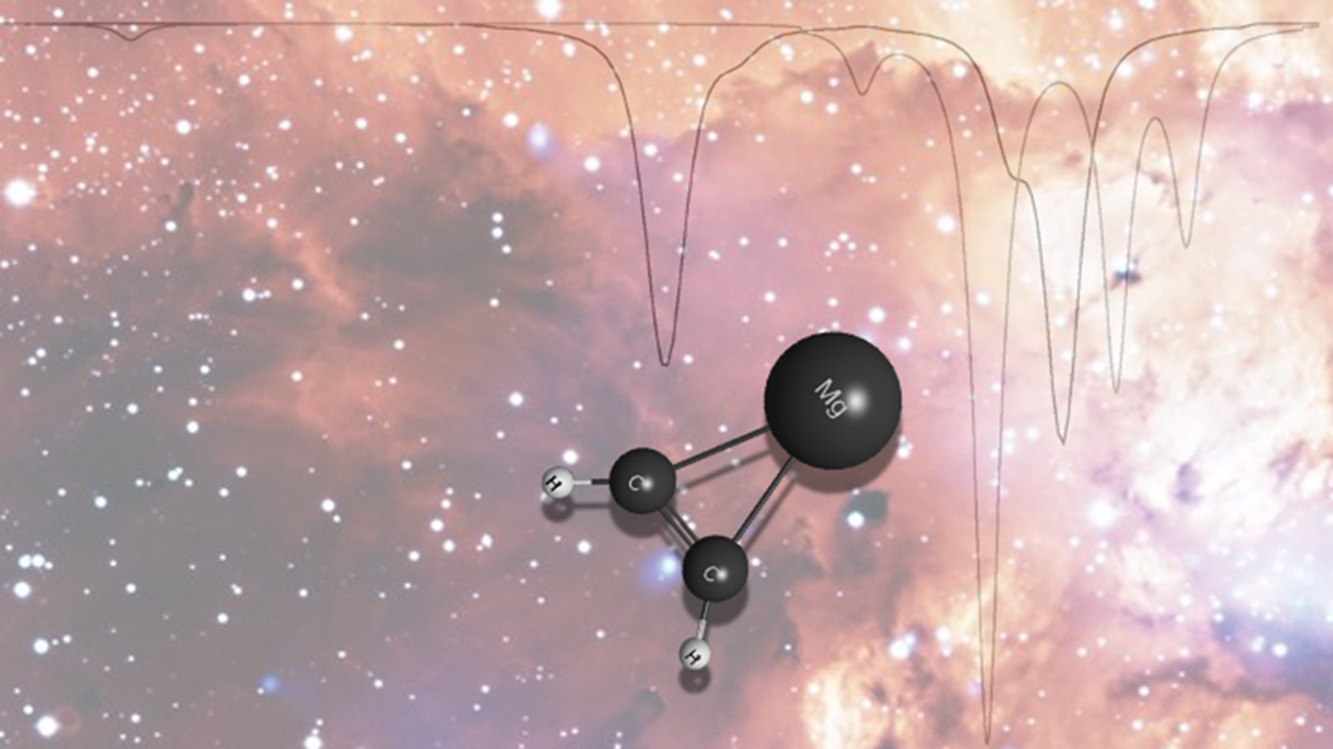
K. N. Poland, C. Z. Palmer, A. Chard, S. R. Davis, and R. C. Fortenberry, “On the Formation and Spectral Signatures of Magnesacyclopropene (c-MgC2H2),” Journal of Molecular Spectroscopy, 382, 111514 (2021). DOI:10.1016/j.jms.2021.111514 (CHE-1757888)
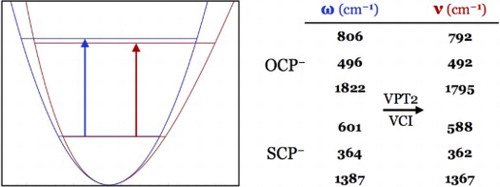
K. R. Barlow, S. M. Goodlett, S. N. Arradondo and G.S. Tschumper, “Fundamental vibrational frequencies of isolated 2-phosphaethynolate and 2-phosphaethynthiolate anions: OCP− and SCP−,” Molecular Physics, e1967495 (2021). DOI: 10.1080/00268976.2021.1967495 (CHE-1757888)
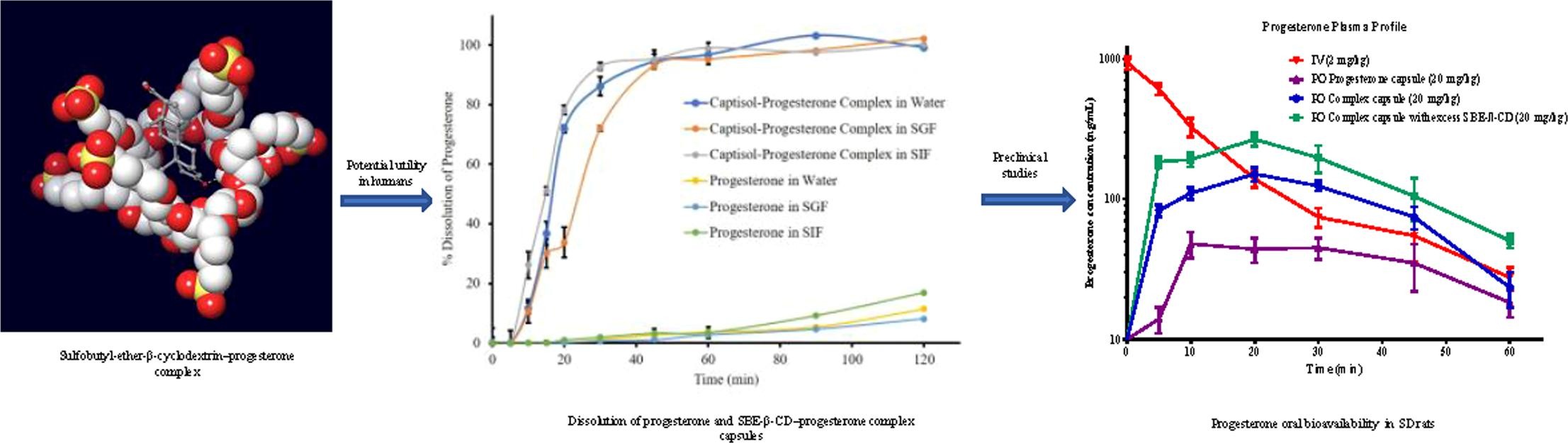
V. K. Shankar, A. Police, P. Pandey, Z. G. Cuny, M. A. Repka, R. J. Doerksen, and S. N. Murthy, “
Optimization of sulfobutyl-ether-β-cyclodextrin levels in oral formulations to enhance progesterone bioavailability,” International Journal of Pharmaceutics 596, 120212 (2021). DOI: 10.1016/j.ijpharm.2021.120212 (CHE-1460568)
H. Shirley, T. M. Sexton, N. P. Liyanage, C. Z. Palmer, L. E. McNamara, N. I. Hammer, G. S. Tschumper, and J. H. Delcamp,“
Effect of “X” Ligands on the Photocatalytic Reduction of CO2 to CO with Re(pyridylNHC-CF3)(CO)3X Complexes, ” European Journal of Inorganic Chemistry, 1844-1851 (2020). DOI: 10.1002/ejic.202000283 (CHE-1757888)
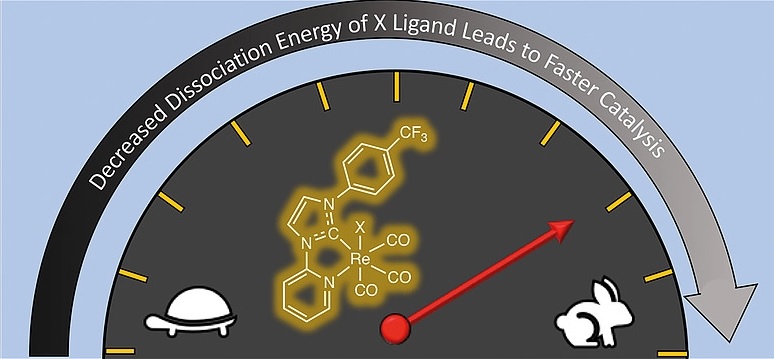
N. Inostroza-Pino, Z. Palmer, T. J. Lee, and R. C.Fortenberry,“
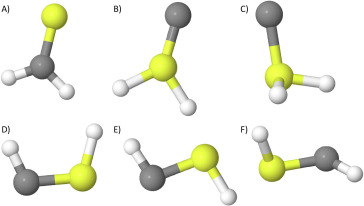
Theoretical rovibrational characterization of the cis/trans-HCSH and H2SC isomers of the known interstellar molecule thioformaldehyde, ” Journal of Molecular Spectroscopy, 369 (2020). DOI: 10.1016/j.jms.2020.111273 (CHE-1757888)
J. Dallas, A. Flint, and R. C. Fortenberry,“
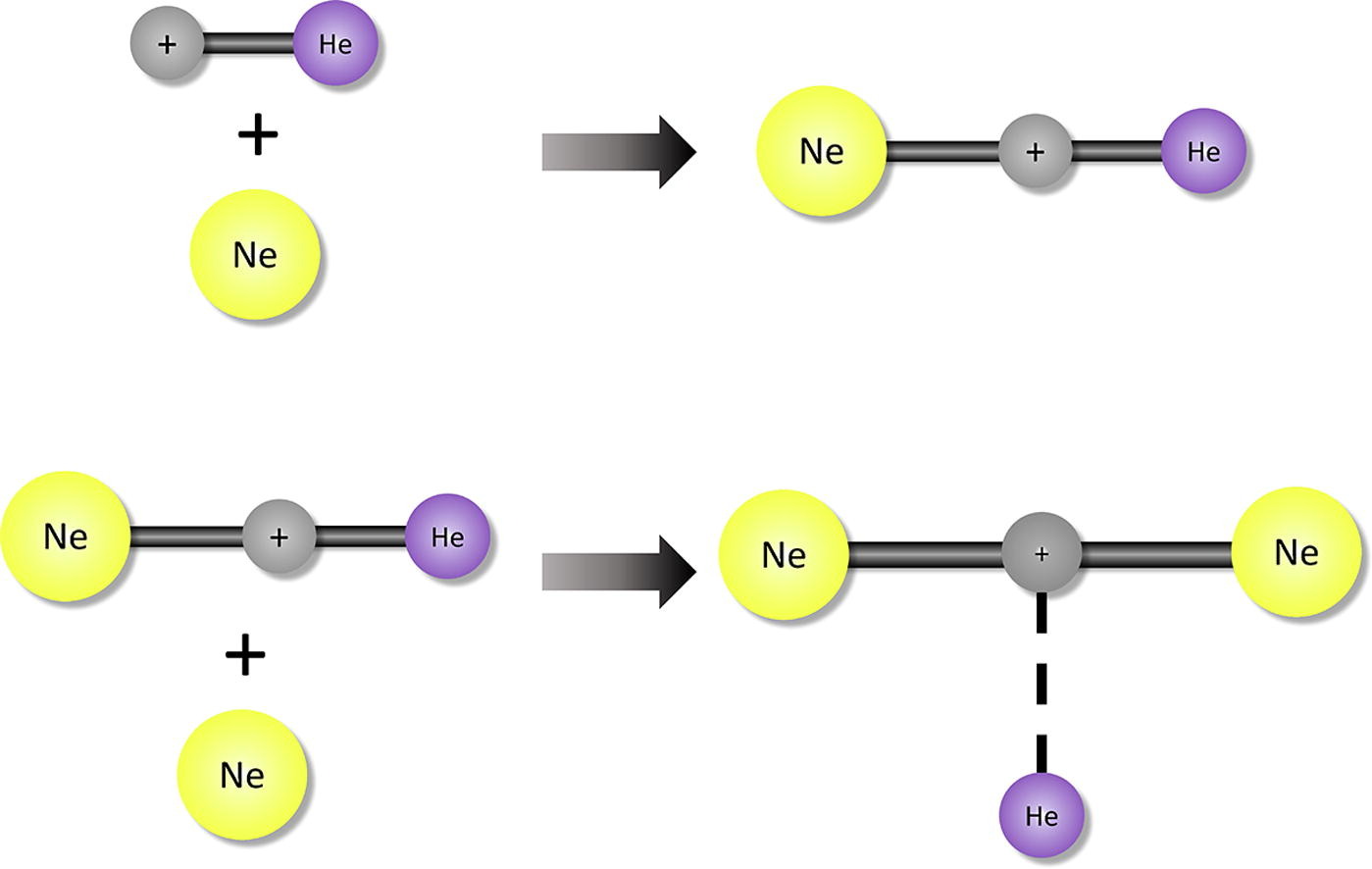
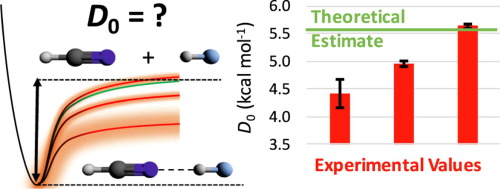
Solvation of HeH+ in neon atoms: Proton-bound complexes of mixed He and Ne, ” Chemical Physics 439, 110927 (2020). DOI: 10.1016/j.chemphys.2020.110927 (CHE-1757888)
T. Sexton, W. Van Benschoten, and G. S. Tschumper, “
Dissociation energy of the HCN⋯HF dimer,” Chemical Physics Letters 748, 137382 (2020). DOI: 10.1016/j.cplett.2020.137382 (CHE-1460568)
A. L. Duddupudi, P. Pandey. H. Vo, C. L. Welsh, R. J. Doerksen, and G. D. Cuny, “
Hypervalent Iodine Mediated Oxidative Cyclization of Acrylamide N-Carbamates to 5,5-Disubstituted Oxazolidine-2,4-diones,” Journal of Organic Chemistry 85, 7549–7557 (2020). DOI: 10.1021/acs.joc.0c00581 (CHE-1460568 & CHE-1757888)
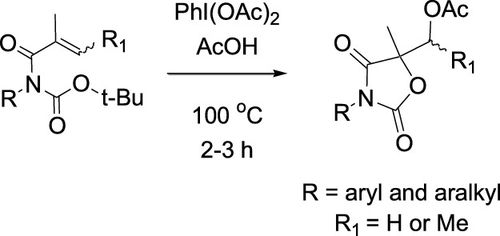
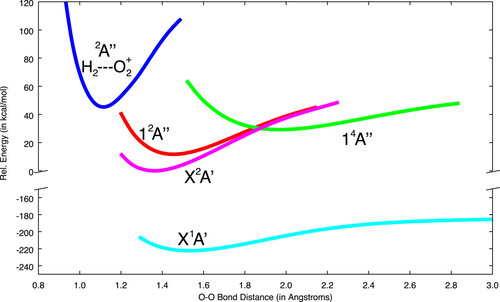
R. C. Fortenberry1, T. Trabelsi, B. R. Westbrook, W. A. Del Rio, and J. S. Francisco, “
Molecular oxygen generation from the reaction of water cations with oxygen atoms,” The Journal of Chemical Physics (2019). DOI: 10.1063/1.5102073 (CHE-1460568)
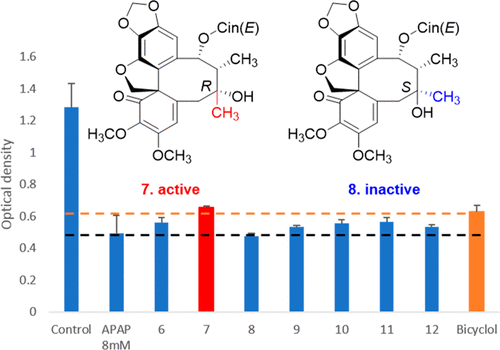
J. Liu, P. Pandey, X. Wang, K. Adams, X. Qi, J. Chen, H. Sun, Q. Hou, D. Ferreira, R. J. Doerksen, S. Li, and M. T. Hamann, “
Hepatoprotective tetrahydrobenzocyclooctabenzofuranone lignans from Kadsura longipedunculata,” Journal of Natural Products 82, 2842-2851 (2019). DOI: 10.1021/acs.joc.0c00581 (CHE-1460568)
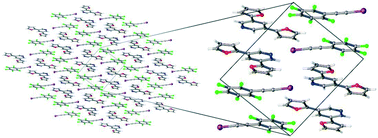
S. N. Johnson, T. L. Ellington, D. T. Ngo, J. L. Nevarez, N. Sparks, A. L. Rheingold, D. L. Watkins, and G. S. Tschumper, “Probing Non-covalent Interactions Driving Molecular Assembly in Organo-electronic Building Blocks,” CrystEngComm (2019). DOI: 10.1039/C9CE00219G (CHE-1460568)
Y. Zou, X. Wang, J. Sims, B. Wang, P. Pandey. C. L. Welsh, R. P. Stone, M. A. Avery, R. J. Doerksen, D. Ferreira, C. Anklin, F. A. Valeriote, M. Kelly, and M. T. Hamann, “Computationally Assisted Discovery and Assignment of a Highly Strained and PANC-1 Selective Alkaloid from Alaska’s Deep Ocean,” Journal of the American Chemical Society, 141, 4338–4344 (2019). DOI: 410.1021/jacs.8b11403 (CHE-1460568)
N. I. Hammer and G. S. Tschumper, “Importance of a Truly Cohesive Theme in a REU Program,” in Best Practices for Chemistry REU Programs, edited by Mark Griep and Linette Watkins, ACS Books, 2018. DOI: 10.1021/bk-2018-1295.ch011

B. R. Westbrook, K. M. Dreux, G. S. Tschumper, J. S. Francisco, and R. C. Fortenberry, “Binding of the atomic cations hydrogen through argon to water and hydrogen sulfide,” Physical Chemistry Chemical Physics, 20, 25967-25973 (2018). DOI: 10.1039/C8CP05378B
S. G. Zetterholm, G. A. Verville, L. Boutwell, C. Boland, J. C. Prather, J. Bethea, J. Cauley, K. Warren, S. A. Smith, D. H. Magers, N. I. Hammer, “Noncovalent Interactions between Tri-methylamine N-oxide (TMAO), Urea, and Water,” Journal of Physical Chemistry B, 122, 8805–8811 (2018). DOI: 10.1021/acs.jpcb.8b04388
S. N. Johnson, C. R. Hutchison‡, C. M. Williams, C. L. Hussey, G. S. Tschumper, and N. I. Hammer, “Intermolecular Interactions and Vibrational Perturbations within Mixtures of 1-Ethyl-3-methylimidazolium Thiocyanate and Water,” Journal of Physical Chemistry C, 122, 27673-27680 (2018). DOI: 10.1021/acs.jpcc.8b07114
Y. Zhang, H. Cheema, A. E. London, A. Morales, J. D. Azoulay and J. H. Delcamp, “Panchromatic cross-conjugated π-bridge NIR dyes for DSCs,” Physical Chemistry Chemical Physics, 20, 2438-2443 (2018). DOI: 10.1039/C7CP06703H
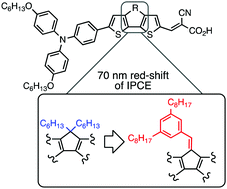
Y. A. Abdo, J. W. Weeks, W. Layfield, W. M. Tremlett, J. W. Graham, M. E. Tabor, S. E. Causey, J. M. Carr, and G. S. Tschumper, “Intramolecular Hydrogen Bonding in α-Epoxy Alcohols: A Conformational Analysis of 1,2-Dialkyl-2,3-epoxycyclopentanol Diastereomers,” Chemistry Letters, 47, 156-159 (2018). DOI: 10.1246/cl.170932
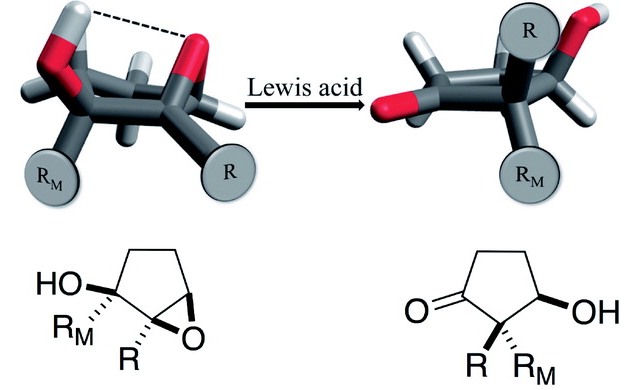
J. D. Veals, K. N. Poland, W. P. Earwood, S. M. Yeager, K. L. Copeland, and S. R. Davis, “MRMP2, CCSD(T), and DFT Calculations of the Isomerization Barriers for the Disrotatory and Conrotatory Isomerizations of 3-Aza-3-ium-dihydrobenzvalene, 3,4-Diaza-3-ium-dihydrobenzvalene, and 3,4-Diaza-diium-dihydrobenzvalene,” Journal of Physical Chemistry A, 121, 8899–8911 (2017). DOI: 10.1021/acs.jpca.7b08227 (CHE-1460568)
N. P. Liyanage, H. Cheema, A. Baumann, A. R. Zylstra, and J. H. Delcamp, “Effect of Donor Strength and Bulk on Thieno[3,4-b]pyrazine based Panchromatic Dyes in DSCs,” ChemSusChem, 10, 2635–2641 (2017). DOI: 10.1002/cssc.201700546
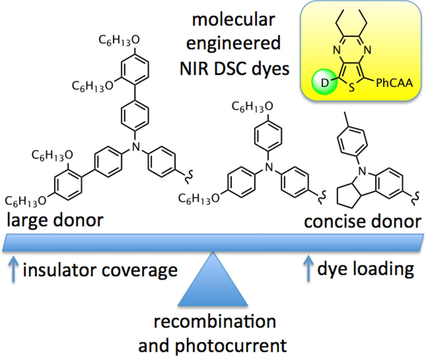
M. Rambukwella, S. Burrage, M. Neubrander, Oscar Baseggio, E. Apra,̀ M. Stener, A. Fortunelli, and A. Dass, “Au38(SPh)24: Au38 Protected with Aromatic Thiolate Ligands,” The Journal of Physical Chemistry Letters, 8, 1530-1537 (2017). DOI: 10.1021/acs.jpclett.7b00193
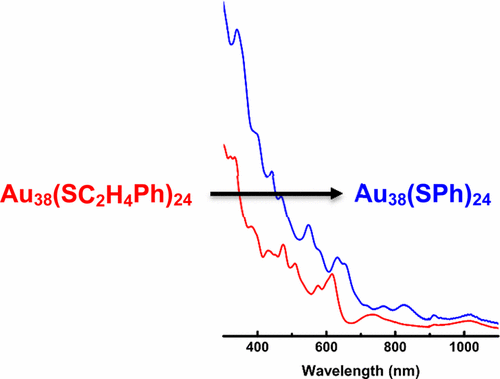
A. J. Huckaba, A. Yella, L. E. McNamara, A. E. Steen, J. S. Murphy, C. A. Carpenter, G. D. Puneky, N. I. Hammer, M. K. Nazeeruddin, M. Grätzel, and J. H. Delcamp, “Molecular Design Principles of Near-Infrared Absorbing and Emitting Indolizine Dyes,” Chemistry - A European Journal, 22, 15536-15542 (2016). DOI: 10.1002/chem.201603165
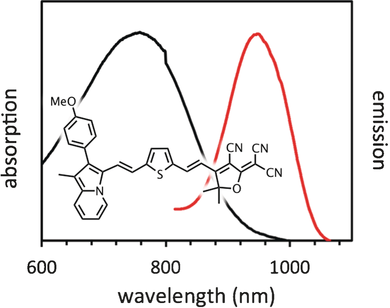
J. T. Kelly, A. K. McClellan, L. V. Joe, A. M. Wright, L. T. Lloyd, G. S.
Tschumper, and N. I. Hammer, “Competition between Hydrophilic and Argyrophilic Interactions in Surface Enhanced Raman Spectroscopy (SERS),” ChemPhysChem, 17, 2782-2786 (2016). DOI: 10.1002/cphc.201600678

J. C. Howard, J. L. Gray, A. J. Hardwick, L. T. Nguyen and G. S. Tschumper, “Getting down to the Fundamentals of Hydrogen Bonding: Anharmonic Vibrational Frequencies of (HF)2 and (H2O)2 from Ab Initio Electronic Structure Computations),” Journal of Chemical Theory and Computation, 10, 5426-5435 (2014). DOI: 10.1021/ct500860v
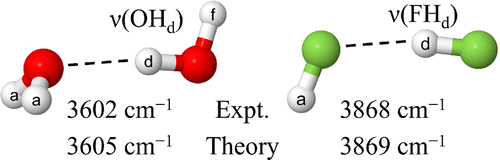
D. N. Reinemann, G. S. Tschumper, and N. I. Hammer, “Characterizing
the B-P Stretching Vibration in Phosphorous Substituted Phosphine Boranes,”
ChemPhysChem, 15, 1867-1871 (2014). DOI: 10.1002/cphc.201400036
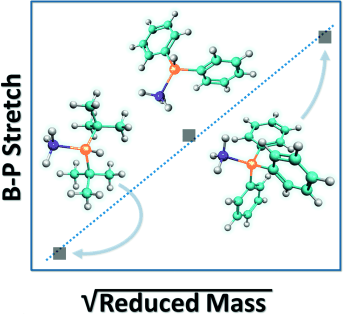
J. Coleman Howard and Gregory S. Tschumper, “Wavefunction methods for the accurate characterization of water clusters,” WIREs Computational Molecular Science, 4, 199–224 (2014). DOI: 10.1002/wcms.1168